Figure 2.
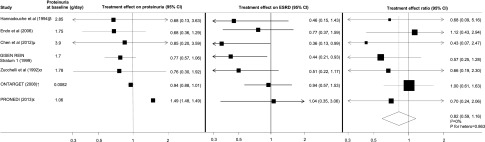
Proteinuria is a promising surrogate for ESRD but data is limited. Comparison of the treatment effects on proteinuria and ESRD. The treatment effect ratio (TER) is the ratio of the relative risks of ESRD and the relative treatment effect on proteinuria; a TER value close to 1 indicates better agreement between the treatment effects. The size of the boxes for the treatment effect ratios (right panel) represent the weight of each study. βauthors have provided final proteinuria data for n=14 in the active (angiotensin-converting enzyme inhibitor [ACEI]) group and n=9 in the control group; µauthors have provided final proteinuria data for n=25 in the active (chelation therapy) group and n=20 in the control group; αauthors have provided final proteinuria data for n=32 in the active (ACEI) group and n=37 in the control group; ↑ONTARGET reported urinary albumin-to-creatinine ratio (UACR; mg/mmol) which has been converted to proteinuria in g/d (UACR mg/mmol was converted to protein excretion mg/d [UACR mg/mmol×10 (10 is the conversion factor)]); εPRONEDI (Progresión de Nefropatía Diabética) reported urinary protein-to-creatinine (UPCR) (g/g), which has been converted to proteinuria in g/d (UPCR g/g was converted to UPCR mg/mmol [UPCR mg/g×0.1131 (0.1131 is the conversion factor)], and UPCR mg/mmol was converted to protein excretion mg/d [UPCR mg/mmol×8.83 (8.83 is the conversion factor)]). Treatment effects on proteinuria are consistent with treatment effects on ESRD. GISEN-REIN, Gruppo Italiano di Studi Epidemiologici in Nefrologia–REIN.
