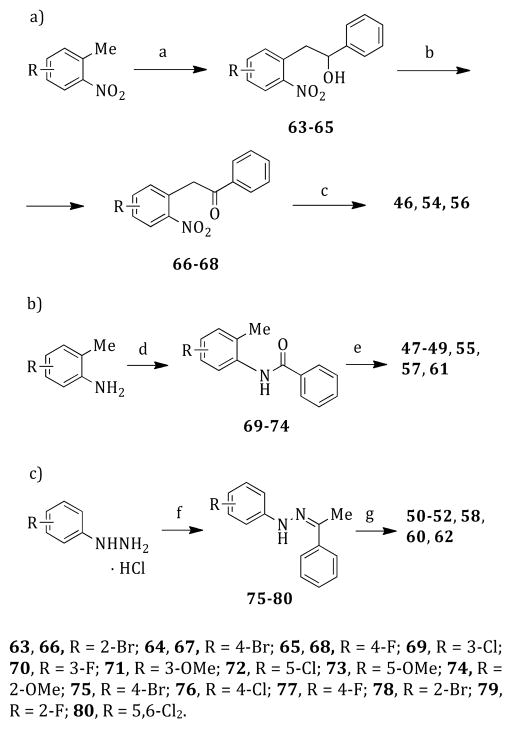
Scheme 2. Synthesis of Intermediates 46–62a.
aReagents and reaction conditions: (a) benzaldehyde, sodium ethoxide, anhydrous DMSO, 25 °C, 12 h, 40–44%; (b) pyridinium chlorochromate, anhydrous dichloromethane, 25 °C, 1.5 h, 16–40%; (c) tin(II) chloride dihydrate/1 N HCl, acetic acid, reflux temperature, 12 h, 19–24%; (d) benzoyl chloride, triethylamine, anhydrous THF, reflux temperature, 2 h, 49–88%; (e) (i) tert-butyllithium, anhydrous THF, −40 °C, Ar stream; (ii) 1 h, 0 °C; (iii) 25 °C, 12 h, 18–52%; (f) acetophenone, sodium acetate, ethanol, open vessel, 80 °C, 250 W, 5 min, cooling-while-heating, 25–95%; (g) polyphosphoric acid, 110 °C, 1 h, 25–82%.