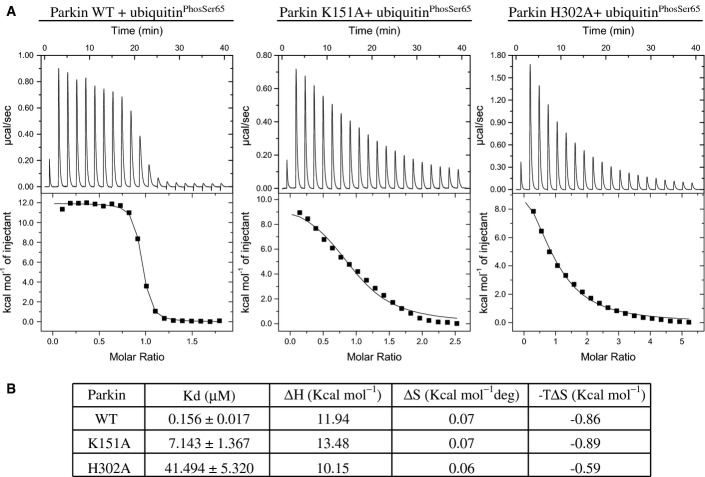
Figure 3.
Parkin His302 and Lys151 are required for optimal binding with ubiquitinPhospho-Ser65
- Parkin H302A and K151A mutants exhibit marked reduction in binding to ubiquitinPhospho-Ser65 compared to wild-type (WT) Parkin. Isothermal calorimetry analysis of Parkin WT (left), K151A mutant (middle) and H302A mutant (right) with ubiquitinPhospho-Ser65.
- Table showing the Kd values (in μM), ΔH values (in kcal/mol), ΔS values (in kcal/mol deg) and −TΔS (in kcal/mol) derived from the graphs. Data are representative of two independent experiments.