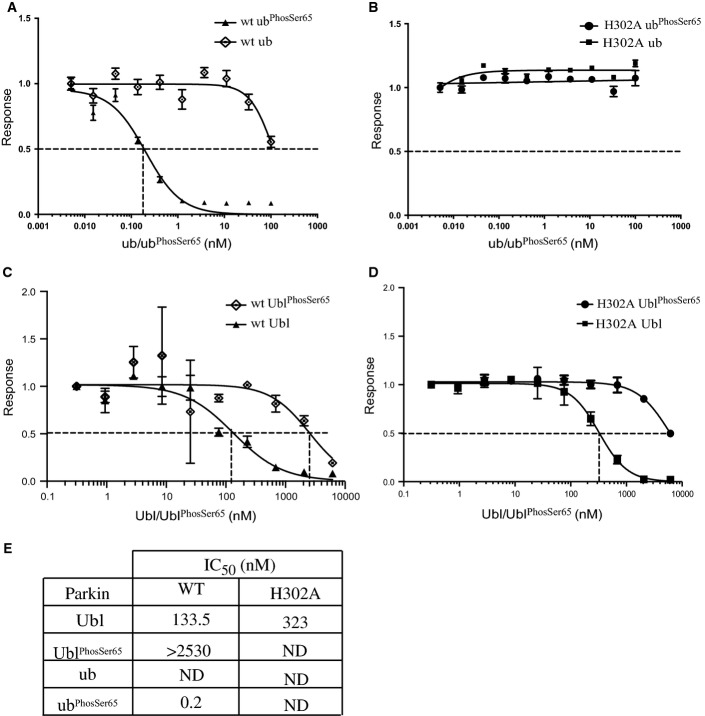
Figure 5.
UbiquitinPhospho-Ser65-mediated disruption of the Ubl domain with ΔUbl Parkin is dependent on residue His302
An AlphaScreen™ binding assay of Parkin Ubl domain and C-terminus of Parkin were established. GST-Ubl (residues 1-76) and wild-type C-terminal Parkin-biotin (residues 80-465) were incubated with streptavidin-coated donor beads and glutathione acceptor beads for 60 min.
- A, B UbiquitinPhospho-Ser65 (ubPhosSer65) disrupts maximal binding signal of Parkin and Ubl interaction of wild-type (wt) (A), but not H302A (H302A) Parkin (B).
- C, D UblPhospho-Ser65 (UblPhosSer65) exhibits substantially reduced ability to disrupt interaction between GST-Ubl and wild-type (C) and H302A Parkin (D).
- E Table showing the IC50 values (in nM) derived from graphs, where the interaction inhibition is incomplete, and not-determined (ND) is indicated.
Data information: Data show mean of one trial ± s.d., n = 3 for each condition (A–D).