Figure 6.
Purified Parkin phosphorylated at Ser65 exhibits constitutive E3 ligase activity that is no longer sensitive to ubiquitinPhospho-Ser65
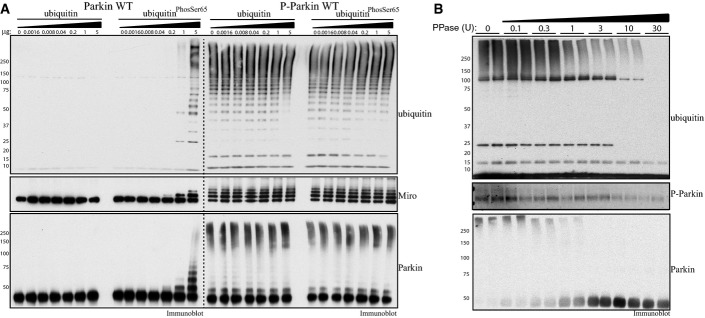
- Parkin phosphorylated at Ser65 exhibits significant constitutive activity. About 2 μg of full-length wild-type (Parkin WT) or phosphorylated at Ser65 (P-Parkin WT) Parkin was analysed using E3 ligase assay with increasing amounts of non-phospho-ubiquitin or ubiquitinPhospho-Ser65 as indicated. Parkin activity was evaluated by immunoblotting as follows: ubiquitin (anti-FLAG-HRP antibody), Parkin (anti-Parkin antibody) and Miro1 (anti-SUMO1 antibody). The molecular mass in kDa is indicated. Data are representative of three independent experiments.
- Dephosphorylation of Parkin at Ser65 leads to reversal of Parkin E3 ligase constitutive activity. About 1 μg of full-length Parkin phosphorylated at Ser65 was subjected to increasing amounts of alkaline phosphatase as indicated. Reactions were then incubated with ubiquitylation assay components (E1 and UbcH7) in the presence of 0.05 mM FLAG-ubiquitin. Reactions were terminated after 60 min by the addition of LDS loading buffer. The effects on Parkin E3 ligase activity were evaluated by ubiquitin chain formation ubiquitylation and Parkin autoubiquitylation evaluated by immunoblotting as follows: ubiquitin (anti-FLAG-HRP antibody) and Parkin (anti-Parkin antibody). The degree of Parkin Ser65 de-phosphorylation was monitored using anti-pSer65 Parkin antibody. Data are representative of four independent experiments.
