Abstract
Background
Adjuvant endocrine therapy (AET) has been shown to reduce the risk of second breast cancer events in women with ductal carcinoma in situ (DCIS). There is no population-level evaluation of AET use in DCIS patients subsequent to standardized reporting of estrogen receptor (ER) status in cancer registries in 2004.
Methods
We conducted a retrospective cohort study of women with DCIS in the National Cancer Database between 2005 and 2012. Patient, tumor and treatment characteristics, and temporal trends associated with receipt of AET were evaluated using generalized linear regression.
Results
Among 206,255 DCIS patients, 36.5% received AET. Fewer than half of ER+ patients (n=62,146, 46.4%) received AET with a modest but significant increase over time (43.6% in 2005 to 47.5% in 2012; unadjusted p-trend <0.001). AET decreased among ER- patients (8.9% to 6.5%, p-trend<0.001). On multivariate analysis, younger (<40 years) and older (≥70 years) women were less likely to receive AET than 50-59 year old women (<40 years RR 0.86, 95% CI 0.82-0.89; ≥70 years, RR 0.79, 95% CI 0.77-0.81). ER+ status conferred a 6.15-fold higher likelihood of receiving AET compared to ER- status (95% CI 5.81-6.50). Women who underwent breast-conserving surgery (BCS) with adjuvant radiation were the most likely to receive AET.
Conclusions
Receipt of AET is relatively low in the group of women most likely to benefit from its use, namely ER+ patients who underwent BCS. Significant variation exists with respect to patient, tumor, site and treatment factors. More tolerable drugs or clearer guideline recommendations may increase use.
Introduction
Ductal carcinoma in situ (DCIS) is a stage 0 breast cancer that accounts for 20% of screen-detected breast malignancies.1 Goals of treatment include prevention of second breast cancer events or evolution into invasive cancer. Standard of care treatment of DCIS includes either breast conserving surgery (BCS) with adjuvant radiation or mastectomy. Approximately 6-30% of women will experience a second breast cancer event after surgical excision of DCIS, at least half of which will be invasive and confer a risk of breast cancer mortality.2-6
Randomized trials have demonstrated the efficacy of tamoxifen as adjuvant endocrine therapy (AET) in preventing second breast events in women with DCIS compared to placebo.7,8 Based on these findings, National Comprehensive Cancer Network guidelines recommend consideration of five years of tamoxifen treatment for patients with DCIS, particularly if estrogen receptor-positive (ER+).9 Exemestane has also been shown to lower the risk of a contralateral breast cancer event after unilateral mastectomy for DCIS, providing another possible adjuvant option for post-menopausal women.10
The heterogeneous potential for invasion and recurrence in DCIS has led to controversy regarding appropriate initial management, and studies have shown marked differences in patterns of care and physician opinions regarding optimal treatment.11 Despite the known benefits of AET, studies have revealed variable levels of acceptance and compliance ranging between 41-66%.12-16 Several of these studies were unable to account for ER status, an important factor in AET treatment. The objective of our study was to conduct a population-level evaluation of trends and characteristics associated with AET use among women with DCIS in a time period that included standardized reporting of ER status in national cancer registries.
Methods
Data Source and Study Cohort
We utilized the National Cancer Database (NCDB), a joint project of the Commission on Cancer, the American College of Surgeons and the American Cancer Society that contains socio-demographic, tumor, treatment and outcome characteristics on approximately 70% of all new cancer diagnoses in the United States annually.17
We retrospectively identified all female patients aged 21 years or older with a diagnosis of unilateral DCIS between 2005 and 2012 (n=284,621). Histologic diagnoses were based on the International Classification of Disease for Oncology, Third Edition (ICD-O-3) codes for DCIS (8201/2, 8230/2, 8500/2, 8503/2, 8507/2, 8523/2, 8501/2). Patients with any previous cancer (n=70,637) were excluded from the analysis, as were patients who did not undergo surgical excision because they could not be distinguished from those diagnosed by autopsy or death (n=7,729). The Institutional Review Board of the Fred Hutchinson Cancer Research Center approved this retrospective study.
Study Variables
The primary outcome, receipt of AET within the first year after DCIS diagnosis, was categorized as a multi-level categorical variable for descriptive purposes, and as a binary variable indicating receipt or non-receipt of AET for trend and multivariate analyses. The categorical variable was characterized as follows: AET not planned as part of therapy; received AET;AET not administered due to contraindication;AET recommended but not administered (no reason provided);AET recommended but not administered (patient refused); and AET recommended, unknown if given. AET non-receipt for the binary variable included patients who did not have AET planned as part of therapy, and those who were recommended AET but did not receive it for any of the above reasons. Other study covariates included patient demographics, tumor characteristics, site characteristics and treatment factors as shown in Table 1.
Facility locations were categorized into regions according to the 2000 United States Census: Northeast, Southeast, Atlantic, Great Lakes, South, Midwest, West, Mountain and Pacific (Figure 2). Facility types were defined as community cancer program (100 to 500 newly diagnosed cases/year), comprehensive community cancer program (≥500 newly diagnosed cases/year) and academic / research program (≥500 newly diagnosed cases/year and participate in physician education and research). A multi-level categorical treatment variable included BCS without radiation, BCS with radiation, unilateral mastectomy and bilateral mastectomy.
Statistical Analysis
STATA/SE 12.1 (StataCorp LP, College Station Texas) was used for all analyses. Descriptive statistics were examined overall and by AET status. Patient demographic, tumor, site and treatment characteristics were compared univariately between patients who received or did not receive AET using Chi-squared tests. All factors were significantly associated with receipt of AET and were included in multivariate estimates. Because the dataset was only 63% complete for all variables, missing values for all study covariates listed in Table 1 were estimated using multiple imputation with chained equations over ten iterations.18 Regression analyses used the combined results of the ten imputations to create valid statistical inferences that account for biases in estimates associated with missing data.19 Patients with a contraindication for AET (n=4,063) were excluded. Because receipt of AET was not expected to be a rare event, we estimated relative risks using generalized linear models with a log-link function, specifying a Poisson distribution and clustering on NCDB facility site.20 Two-sided p-values of <0.05 were considered statistically significant.
Results
Demographics, Tumor and Treatment Characteristics
We identified 206,255 patients diagnosed with unilateral DCIS between 2005 and 2012. The median age was 58 years, and the majority of patients were white (81.8%), had no comorbid conditions (87.9%), were privately insured (63.3%) and received treatment at a comprehensive community cancer program (60.0%) (Table 1). Ninety-six percent had negative margins. Half of patients underwent BCS with radiation; fewer than 10% underwent bilateral mastectomy.
Table 1.
Patient demographic, tumor, site and treatment characteristics among women with ductal carcinoma in situ, 2005-2012.
| No endocrine therapy use (n=122,554) | Endocrine therapy use (n=70,302) | p-value | |
|---|---|---|---|
|
|
|
||
| Characteristic | N (%) | N (%) | |
| Patient Demographics | |||
| Age, years | <0.001 | ||
| Median years [IQRa] | 59 [49-69] | 57 [49-65] | |
| <40 | 4,519 (3.7) | 1,537 (2.2) | |
| 40-49 | 26,146 (21.3) | 16,258 (23.1) | |
| 50-59 | 33,244 (27.1) | 22,872 (32.5) | |
| 60-69 | 30,137 (24.6) | 18,875 (26.8) | |
| ≥70 | 28,508 (23.3) | 10,760 (15.3) | |
| Year of diagnosis | <0.001 | ||
| 2005 | 13,744 (11.2) | 6,688 (9.5) | |
| 2006 | 14,081 (11.5) | 7,740 (11.0) | |
| 2007 | 14,837 (12.1) | 8,460 (12.0) | |
| 2008 | 15,668 (12.8) | 8,908 (12.7) | |
| 2009 | 16,517 (13.5) | 9,331 (13.3) | |
| 2010 | 15,777 (12.9) | 9,126 (13.0) | |
| 2011 | 15,992 (13.0) | 9,897 (14.1) | |
| 2012 | 15,938 (13.0) | 10,152 (14.4) | |
| Race/ethnicity | <0.001 | ||
| White | 101,125 (82.5) | 57,123 (81.3) | |
| Black | 13,820 (11.3) | 8,977 (12.8) | |
| American Indian/Alaska Native | 241 (0.2) | 131 (0.2) | |
| Asian/Pacific Islander | 4,758 (3.9) | 2,752 (3.9) | |
| Other | 2,610 (2.1) | 1,319 (1.9) | |
| Charlson-Deyo Score | <0.001 | ||
| 0 | 107,266 (87.5) | 62,073 (88.3) | |
| 1 | 12,863 (10.5) | 7,148 (10.2) | |
| 2+ | 2,425 (2.0) | 1,081 (1.5) | |
| Primary payor | <0.001 | ||
| Private insurance | 73,435 (61.1) | 46,251 (66.7) | |
| Medicaid | 5,937 (4.9) | 4,075 (5.9) | |
| Medicare | 39,017 (32.4) | 17,787 (25.7) | |
| Uninsured | 1,874 (1.6) | 1,195 (1.7) | |
| Tumor characteristics | |||
| Grade | <0.001 | ||
| Well differentiated | 15,062 (15.4) | 10,277 (18.0) | |
| Moderately differentiated | 37,373 (38.2) | 25,211 (44.2) | |
| Poorly differentiated | 40,578 (41.5) | 19,585 (34.4) | |
| Undifferentiated | 4,719 (4.8) | 1,938 (3.4) | |
| Estrogen receptor status | <0.001 | ||
| ER-a | 24,327 (24.5) | 1,897 (3.0) | |
| ER+ | 74,994 (75.5) | 62,146 (97.0) | |
| Site and treatment characteristics | |||
| Facility type | <0.001 | ||
| Community cancer program | 12,143 (9.9) | 8,184 (11.6) | |
| Comprehensive community cancer program | 74,305 (60.6) | 42,027 (59.8) | |
| Academic/research program | 35,930 (29.3) | 19,974 (28.4) | |
| Other | 176 (0.1) | 117 (0.2) | |
| Facility location | <0.001 | ||
| Northeast | 7,695 (6.3) | 5,295 (7.5) | |
| Atlantic | 17,954 (14.6) | 11,509 (16.4) | |
| Southeast | 28,547 (23.3) | 15,622 (22.2) | |
| Great Lakes | 18,644 (15.2) | 15,331 (21.8) | |
| South | 7,611 (6.2) | 3,410 (4.9) | |
| Midwest | 8,185 (6.7) | 5,446 (7.7) | |
| West | 10,335 (8.4) | 4,061 (5.8) | |
| Mountain | 5,803 (4.7) | 2,645 (3.8) | |
| Pacific | 17,780 (14.5) | 6,983 (9.9) | |
| Final margin status | <0.001 | ||
| Negative | 115,306 (96.1) | 67,174 (96.7) | |
| Positive | 4,678 (3.9) | 2,293 (3.3) | |
| Treatment | <0.001 | ||
| BCSa only | 30,384 (24.9) | 8,368 (11.9) | |
| BCS with radiation | 48,939 (40.1) | 52,221 (74.4) | |
| Unilateral mastectomy | 28,130 (23.0) | 8,689 (12.4) | |
| Bilateral mastectomy | 14,685 (12.0) | 922 (1.3) | |
BCS, breast-conserving surgery; ER, estrogen receptor; IQR, interquartile range.
ER testing was not performed or results were not available for 29.2% of patients in 2005, but this decreased to 7% by 2012. During this timeframe, the proportion of patients reported as ER+ increased from 57.9% to 80.5% (data not shown). The proportion of patients who underwent BCS without radiation decreased from 23.7% in 2005 to 21.0% in 2012 (unadjusted p-trend <0.001), whereas BCS with radiation increased non-significantly from 49.9% to 51.0% (unadjusted p-trend=0.39). Bilateral mastectomies almost doubled from 5.2% in 2005 to 10.0% in 2012 (unadjusted p-trend <0.001). All other covariates were significantly associated with study time, but without discernable patterns.
Trends in and Factors Associated with Receipt of AET
Overall, 36.5% of patients received AET, with an increase from 33.1% in 2005 to 40.0% in 2012 (unadjusted p-trend <0.001) (Figure 1). Contraindications were rare (n=4,063, 2.1%), as was patient refusal of AET following recommendation by a treating physician (n=14,001, 7.1%). Among ER+ patients, fewer than half (n=62,146, 46.4%) received AET. There was a modest but significant increase over time from 43.6% in 2005 to 47.5% in 2012 (unadjusted p-trend <0.001). Conversely, the proportion of ER- patients who received AET decreased during the same time interval from 8.9% to 6.5% (unadjusted p-trend <0.001). Temporal trends observed for AET use among ER+ and ER- patients remained significant in multivariate analysis after adjusting for all time-varying covariates.
Figure 1.
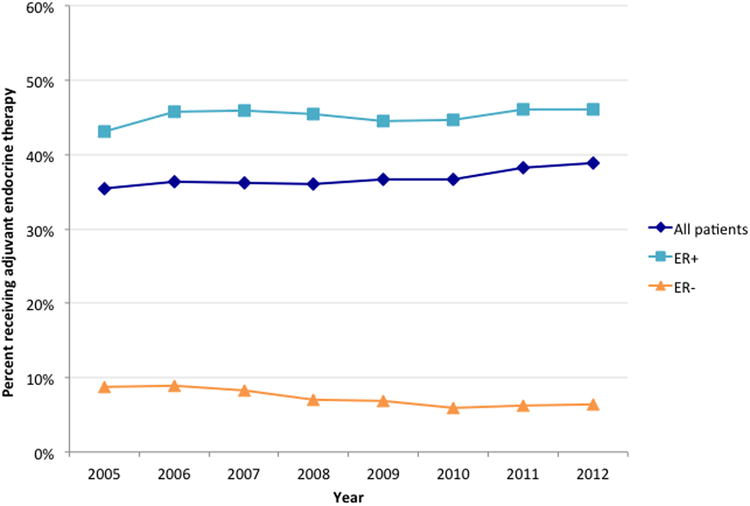
Overall and estrogen-receptor stratified trends in receipt of adjuvant endocrine therapy among patients without contraindications.
The proportion of patients who received AET according to patient demographic, tumor, site and treatment characteristics are summarized in Table 1. In univariate analyses, patients in the youngest (<40 years) and oldest (≥70 years) age groups were least likely to receive AET. Characteristics associated with a higher likelihood of receiving AET were well or moderately differentiated DCIS, ER+ status, black race, treatment at a community cancer program, or treatment in the Northeast, Atlantic, Great Lakes or Midwest. Negative pathologic margins and BCS followed by adjuvant radiation were also associated with receipt of AET.
In multivariate analyses, numerous factors remained independently associated with receipt of AET (Table 2). Women aged 50-59 years were the most likely to receive AET whereas women <40 years and ≥70 years were least likely. Women with ER+ DCIS were 6.15 times more likely to receive AET than women with ER- DCIS (95% CI 5.81-6.50). Compared to patients with positive margins or well-differentiated tumors, those with negative margins or poorly differentiated tumors were slightly more likely to receive AET. Significant variation was also noted by location, with patients in the western part of the United States and Texas, Oklahoma, Louisiana and Arkansas 14-24% less likely to receive AET than women in the northeast (Figure 2). Likewise, variation existed by type of surgical treatment (Figure 3). Women who underwent BCS alone, unilateral mastectomy, or bilateral mastectomy were significantly less likely to receive AET compared to women who underwent BCS followed by adjuvant radiation.
Table 2.
Multivariate analysis of factors associated with adjuvant endocrine therapy use among women with ductal carcinoma in situ, 2005-2012.
| Receipt of adjuvant endocrine therapy | |||
|---|---|---|---|
|
|
|||
| Patient Characteristics | aRRa | 95% CIa | p-value |
| Demographic characteristics | |||
| Age Groups | |||
| <40 | 0.86 | 0.82-0.89 | <0.001 |
| 40-49 | 0.98 | 0.97-1.00 | 0.01 |
| 50-59 | 1.0 | reference | reference |
| 60-69 | 0.96 | 0.95-0.98 | <0.001 |
| ≥70 | 0.79 | 0.77-0.81 | <0.001 |
| Year of Diagnosis | |||
| 2005 | 1.0 | reference | reference |
| 2006 | 1.06 | 1.03-1.09 | <0.001 |
| 2007 | 1.08 | 1.05-1.12 | <0.001 |
| 2008 | 1.09 | 1.06-1.12 | <0.001 |
| 2009 | 1.08 | 1.05-1.12 | <0.001 |
| 2010 | 1.10 | 1.06-1.14 | <0.001 |
| 2011 | 1.14 | 1.10-1.18 | <0.001 |
| 2012 | 1.17 | 1.13-1.21 | <0.001 |
| Race/ethnicity | |||
| White | 1.0 | reference | reference |
| Black | 1.04 | 1.00b-1.07 | 0.025 |
| American Indian/Alaska Native | 0.99 | 0.87-1.12 | 0.817 |
| Asian/Pacific Islander | 1.07 | 1.02-1.11 | 0.003 |
| Other | 0.94 | 0.89-0.99 | 0.015 |
| Charlson-Deyo Score | |||
| 0 | 1.0 | reference | reference |
| 1 | 1.03 | 1.00-1.05 | 0.018 |
| 2+ | 0.98 | 0.93-1.02 | 0.307 |
| Primary Payor | |||
| Private insurance | 1.0 | reference | reference |
| Medicaid | 1.06 | 1.03-1.09 | <0.001 |
| Medicare | 0.94 | 0.93-0.96 | <0.001 |
| Uninsured | 1.04 | 0.98-1.11 | 0.166 |
| Tumor characteristics | |||
| Grade | |||
| Well differentiated | 1.0 | reference | reference |
| Moderately differentiated | 1.01 | 0.99-1.03 | 0.464 |
| Poorly differentiated | 1.02 | 1.00-1.05 | 0.015 |
| Undifferentiated | 1.03 | 0.98-1.08 | 0.278 |
| Estrogen receptor status | |||
| ER- a | 1.0 | reference | reference |
| ER+ | 6.15 | 5.81-6.50 | <0.001 |
| Site and treatment characteristics | |||
| Facility type | |||
| Community cancer program | 1.0 | reference | reference |
| Comprehensive community cancer program | 1.07 | 1.02-1.12 | 0.004 |
| Academic/Research program | 0.95 | 0.90-1.01 | 0.08 |
| Other | 0.90 | 0.86-0.94 | <0.001 |
| Facility Location | |||
| Northeast | 1.0 | reference | reference |
| Atlantic | 1.01 | 0.92-1.10 | 0.861 |
| Southeast | 0.94 | 0.86-1.03 | 0.164 |
| Great Lakes | 1.10 | 1.02-1.19 | 0.014 |
| South | 0.87 | 0.76-1.01 | 0.063 |
| Midwest | 1.03 | 0.93-1.13 | 0.584 |
| West | 0.82 | 0.73-0.91 | <0.001 |
| Mountain | 0.86 | 0.75-0.98 | 0.027 |
| Pacific | 0.76 | 0.69-0.83 | <0.001 |
| Final margin status | |||
| Negative | 1.0 | reference | reference |
| Positive | 0.96 | 0.93-0.99 | 0.023 |
| Combined treatment | |||
| BCSa only | 0.43 | 0.41-0.45 | <0.001 |
| BCS with radiation | 1.0 | reference | reference |
| Unilateral mastectomy | 0.51 | 0.49-0.53 | <0.001 |
| Bilateral mastectomy | 0.12 | 0.11-0.13 | <0.001 |
aRR, adjusted relative risk; CI, confidence interval; ER, estrogen receptor; BCS, breast conservation surgery.
Confidence intervals with significant p-values (<0.05) and an upper or lower confidence level of 1.00 were rounded from 0.999; confidence intervals for these estimates do not include 1.0.
Figure 2.
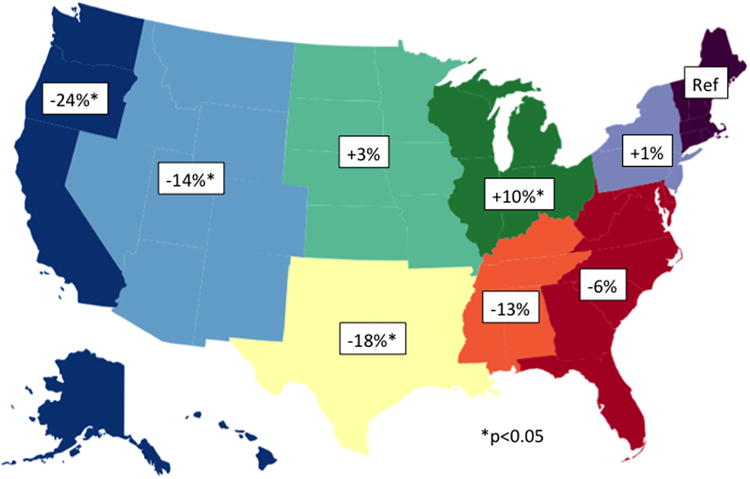
Multivariate adjusted relative likelihood of receipt of adjuvant endocrine therapy compared to Northeast, according to geographic location of treatment.
Figure 3.
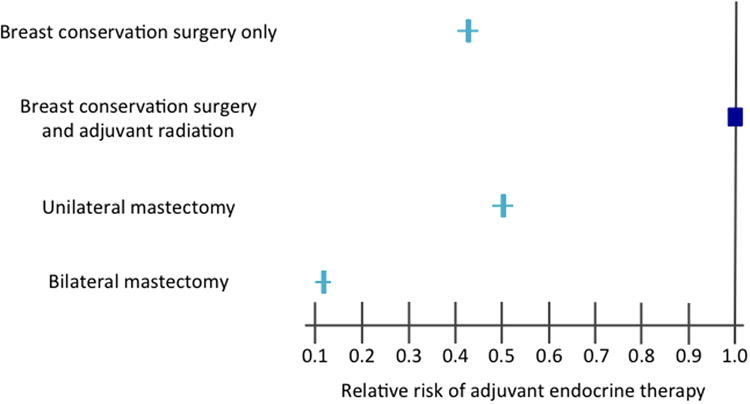
Multivariate adjusted relative risk of receipt of adjuvant endocrine therapy according to treatment type.
Discussion
Randomized controlled trials have demonstrated 31-66% reduced relative risk of second breast cancer events among women who received AET for DCIS.7,8,10,21,22 Despite this proven benefit, studies have shown highly variable AET use among DCIS patients, ranging from a low of 15% to a high of 73%.12-16,23-25 To our knowledge, this is the first population-based study of AET for DCIS in the contemporary era of standardized ER reporting. The results of our study indicate that among women who can expect the greatest potential AET benefit, those with ER+ DCIS, only 46% received AET. Reassuringly, we found that only 3% of patients who received AET were ER-. This finding, coupled with the fact that there was a significant decrease in the proportion of patients for whom ER testing was not performed or not available (29% in 2005 to 7% in 2012), suggests that immunohistochemical ER staining has become widely adopted for DCIS, and that test results are appropriately guiding clinical management.
Type of initial treatment was significantly associated with receipt of AET. Not surprisingly, women who underwent bilateral mastectomy were least likely to receive AET, as there is no evidence that AET benefits this population. However, given the National Surgical Breast and Bowel Project (NSABP) B-06 and B-17 trial results that demonstrated higher local recurrence rates for BCS alone compared to either mastectomy or BCS with adjuvant radiation,26,27 respectively, we found it very surprising that patients undergoing BCS alone were less likely to receive AET. Likewise, despite a two to three times increased risk of local recurrence with positive pathologic margins,27,28 these women were significantly less likely to receive AET than those with negative margins. Possible explanations for these findings may be that patients who chose to undergo mastectomy for DCIS harbored more extensive disease, had strong family histories or increased anxiety regarding recurrence. These patients may have been more inclined to accept elective AET as chemoprevention. Conversely, it may be that the physicians of or patients who elect to undergo BCS without radiation, or those who do not have further surgery for positive margins consciously chose to treat DCIS less aggressively by forgoing AET.
Patient age was also significantly associated with AET receipt. Women who were younger (<40 years) and older (≥70 years) were least likely to receive AET. Decreased utilization in the younger age group may be explained by the fact that tamoxifen administration is not compatible with childbearing, and aromatase inhibitors are not recommended in pre-menopausal women with intact ovarian function.29 All AET scan cause undesirable side effects, including hot flashes and vaginal dryness,30 and tamoxifen carries a 2 to 4-fold increased risk of endometrial cancer and 2-fold increased risk of venous thromboemobolism (VTE).31 Previous studies have also shown a decline in receipt of AET with increasing age,24,25 which may be due to concerns of heightened risk of VTE and endometrial cancer in older women.32 It may also reflect an acceptance of findings from studies that demonstrated acceptably low breast cancer recurrence and mortality rates with the omission of select aspects of adjuvant breast cancer treatment in elderly women with early stage invasive breast cancer.33,34
There is considerable controversy regarding optimal treatment of DCIS given its heterogeneity and survivability.23-25,35 Some clinicians have even advocated eliminating the use of the term “carcinoma” from the description of DCIS.36 It is not surprising, therefore, that we found wide variation in receipt of AET across geographic location and treatment facility type. A survey of DCIS management among radiation oncologists demonstrated substantial differences in the likelihood of recommendation of adjuvant tamoxifen in DCIS patients. Fifty-six percent of survey respondents stated they would always recommend, 20% if additional breast cancer risk factors, 11% if family history, and 6% very rarely or never.11 Studies evaluating AET initiation point to the critical role of physicians. In a study of invasive cancer patients, 63% of patients who did not initiate AET cited ‘clinician factors’ as the primary reason. These included lack of adequate information about side effects and allowing for independent patient decision-making.37 Our finding that only 7% of women refused AET when it was recommended substantiates this claim. Similar results are seen in studies including only DCIS patients. Physician recommendation for ‘necessary’ rather than ‘optional’ AET treatment in DCIS has been associated with 11-fold increased odds of use,12 suggesting that one of the primary factors associated with AET acceptance is how AET is presented to patients -- as chemoprevention or necessary treatment.
One of the main strengths of this study is that the NCDB is estimated to capture approximately 70% of all new DCIS diagnoses, and thus is an informative reflection of nationwide practice patterns. However, there are limitations to our study, including our inability to assess patient or physician level decision-making regarding recommendations for and acceptance of AET. We were unable to assess AET compliance, which limits our ability to extrapolate to long-term AET use. Additionally, the NCDB does not provide information on the specific type of AET being offered to patients, family history or BRCA status, all of which may have affected a patient's likelihood of acceptance. Misclassification based on chart abstraction is possible, but data abstractors have a rigorous process by which they classify and follow-up with patients, so any misclassification biases should be minimal.
This study demonstrates that receipt of AET among patients with DCIS remains low in the modern era, and variability exists according to patient, tumor and treatment characteristics. Absolute decreases in risk remain modest, and the low breast cancer-related mortality associated with DCIS (<5% at 15 years) is not changed with the addition of adjuvant AET.27 In the context of serious concerns about potential side effects and health risks, it remains challenging to convince clinicians and patients that the benefits of AET outweigh the risks. Regardless of these drawbacks, AET has been shown to decrease second invasive breast cancer events, which should not be dismissed. There are also substantial risks associated with breast cancer treatment, not to mention increased patient anxiety and decreased quality of life. Individualized risk-benefit discussions should be pursued with all patients. Use of aromatase inhibitors, now the standard of care for AET among postmenopausal women with invasive breast cancer, is a potential option for patients with DCIS though not currently FDA approved for this indication. The recent IBIS-II and NCIC Clinical Trials Group MAP.3 prospective randomized trials demonstrated between 50% and 65% relative reduction in invasive breast cancer among high risk post-menopausal women taking anastrazole or exemastane, respectively, compared to placebo.10,22 Contraindications associated with use are fewer for aromatase inhibitors compared to tamoxifen,29 and DCIS patients have shown improved long-term compliance with aromatase inhibitors.16 The ability to offer patients a wider variety of adjuvant treatment options with different, and possibly more tolerable side effect profiles, is one aspect that could potentially improve acceptance of AET. Our findings also serve to highlight the need for better consensus and clearer national guidelines regarding the role of AET in the treatment of DCIS patients.
Synopsis.
Despite clinical trial evidence that adjuvant endocrine therapy significantly reduces the risk of a second breast cancer event in women with DCIS, less than 50% of estrogen receptor positive (ER+) eligible women receive treatment.
Acknowledgments
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Financial support: MRF was supported by a T32 grant support from the National Cancer Institute under Award Number CA009168. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure statement: The authors report no conflict of interest.
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA A Cancer Journal for Clinicians. 2014;64(1):52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Hughes LL, Wang M, Page DL, et al. Local Excision Alone Without Irradiation for Ductal Carcinoma In Situ of the Breast: A Trial of the Eastern Cooperative Oncology Group. Journal of Clinical Oncology. 2009;27(32):5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wehner P, Lagios MD, Silverstein MJ. DCIS treated with excision alone using the National Comprehensive Cancer Network (NCCN) guidelines. Ann Surg Oncol. 2013;20(10):3175–3179. doi: 10.1245/s10434-013-3176-2. [DOI] [PubMed] [Google Scholar]
- 4.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. JNCI Journal of the National Cancer Institute. 2011;103(6):478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee LA, Silverstein MJ, Chung CT, et al. Breast cancer–specific mortality after invasive local recurrence in patients with ductal carcinoma-in-situ of the breast. The American Journal of Surgery. 2006;192(4):416–419. doi: 10.1016/j.amjsurg.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Falk RS, Hofvind S, Skaane P, Haldorsen T. Second events following ductal carcinoma in situ of the breast: a register-based cohort study. Breast Cancer Res Treat. 2011;129(3):929–938. doi: 10.1007/s10549-011-1531-1. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12(1):21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. The Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger D, Akerley W, Borghaie H, Chang A, Cheney R. NCCN Clinical Practice Guidelines in Oncology. 2nd. National Comprehensive Cancer Network; 2013. pp. 1–138. [Google Scholar]
- 10.Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 11.Ceilley E, Jagsi R, Goldberg S, Kachnic L, Powell S, Taghian A. The management of ductal carcinoma in situ in North America and Europe. Results of a survey. Cancer. 2004;101(9):1958–1967. doi: 10.1002/cncr.20580. [DOI] [PubMed] [Google Scholar]
- 12.Livaudais JC, Hwang ES, Karliner L, et al. Adjuvant Hormonal Therapy Use Among Women with Ductal Carcinoma In Situ. Journal of Women's Health. 2012;21(1):35–42. doi: 10.1089/jwh.2011.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakhlis F, Lazarus L, Hou N, et al. Tamoxifen use in patients with ductal carcinoma in situ and T1a/b N0 invasive carcinoma. ACS. 2005;201(5):688–694. doi: 10.1016/j.jamcollsurg.2005.06.195. [DOI] [PubMed] [Google Scholar]
- 14.Yen TWF, Kuerer HM, Ottesen RA, et al. Impact of randomized clinical trial results in the national comprehensive cancer network on the use of tamoxifen after breast surgery for ductal carcinoma in situ. J Clin Oncol. 2007;25(22):3251–3258. doi: 10.1200/JCO.2006.10.2699. [DOI] [PubMed] [Google Scholar]
- 15.Hird RB, Chang A, Cimmino V, et al. Impact of estrogen receptor expression and other clinicopathologic features on tamoxifen use in ductal carcinoma in situ. Cancer. 2006;106(10):2113–2118. doi: 10.1002/cncr.21873. [DOI] [PubMed] [Google Scholar]
- 16.Virnig BA, Torchia MT, Jarosek SL, Durham S, Tuttle TM. Use of Endocrine Therapy Following Diagnosis of Ductal Carcinoma in Situ or Early Invasive Breast Cancer: Data Points # 14. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011. [PubMed] [Google Scholar]
- 17.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schafer J. Analysis of Incomplete Multivariate Data. London: Chapman and Hall; 1997. [DOI] [Google Scholar]
- 19.Rubin DB. Inference and missing data. Biometrika. 1976 doi: 10.1093/biomet/63.3.581. [DOI] [Google Scholar]
- 20.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 21.Allred DC, Anderson SJ, Paik S, et al. Adjuvant Tamoxifen Reduces Subsequent Breast Cancer in Women With Estrogen Receptor-Positive Ductal Carcinoma in Situ: A Study Based on NSABP Protocol B-24. Journal of Clinical Oncology. 2012;30(12):1268–1273. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383(9922):1041–1048. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 23.Jackson LC, Camacho F, Levine EA, Anderson RT, Stewart JH. Patterns of care analysis among women with ductal carcinoma in situ in North Carolina. Am J Surg. 2008;195(2):164–169. doi: 10.1016/j.amjsurg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Feigelson HS, Carroll NM, Weinmann S, et al. Treatment patterns for ductal carcinoma in situ from 2000-2010 across six integrated health plans. Springer Plus. 2015;4(1):24. doi: 10.1186/s40064-014-0776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haque R, Achacoso NS, Fletcher SW, et al. Treatment of ductal carcinoma in situ among patients cared for in large integrated health plans. Am J Manag Care. 2010;16(5):351–360. [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher ER, Sass R, Fisher B, Wickerham L, Paik SM. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol 6). I. Intraductal carcinoma (DCIS) Cancer. 1986;57(2):197–208. doi: 10.1002/1097-0142(19860115)57:2<197∷AID-CNCR2820570203>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Wapnir IL, Dignam JJ, Fisher B, et al. Long-Term Outcomes of Invasive Ipsilateral Breast Tumor Recurrences After Lumpectomy in NSABP B-17 and B-24 Randomized Clinical Trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solin LJ, Fourquet A, Vicini FA, et al. Long-term outcome after breast-conservation treatment with radiation for mammographically detected ductal carcinoma in situ of the breast. Cancer. 2005;103(6):1137–1146. doi: 10.1002/cncr.20886. [DOI] [PubMed] [Google Scholar]
- 29.Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942–2962. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 30.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. The Lancet. 2003;361(9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 31.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. The Lancet. 1998;351(9114):1451–1467. [PubMed] [Google Scholar]
- 32.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. JNCI Journal of the National Cancer Institute. 2005;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 33.Javid SH, He H, Korde LA, Flum DR, Anderson BO. Predictors and outcomes of completion axillary node dissection among older breast cancer patients. Ann Surg Oncol. 2014;21(7):2172–2180. doi: 10.1245/s10434-014-3595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 35.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in the Treatment of Ductal Carcinoma In Situ of the Breast. JNCI Journal of the National Cancer Institute. 2004;96(6):443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 36.Allegra CJ, Aberle DR, Ganschow P, et al. National Institutes of Health State-of-the-Science Conference Statement: Diagnosis and Management of Ductal Carcinoma In Situ September 22-24, 2009. JNCI Journal of the National Cancer Institute. 2010;102(3):161–169. doi: 10.1093/jnci/djp485. [DOI] [PubMed] [Google Scholar]
- 37.Friese CR, Pini TM, Li Y, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138(3):931–939. doi: 10.1007/s10549-013-2499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


