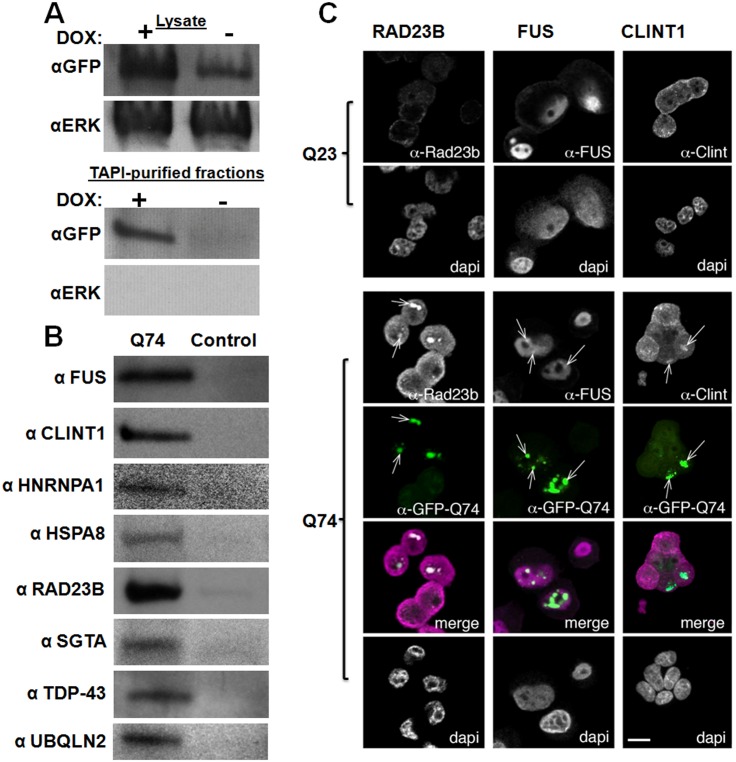
Fig 5. Confirmation of polyQ-associated proteins from PC-12 cells identified by TAPI.
(A) Western blotting shows that the addition of doxycycline to the PC-12 cell model induces the expression of HttQ74-GFP, resulting in aggregates that can be purified by TAPI. The kinase ERK is probed as a negative control; ERK was never identified by mass spectrometry, so is not expected to co-fractionate with polyQ aggregates. (B) Western blot analysis of TAPI-purified polyQ aggregates from PC-12 cells confirms the presence of several disease-associated proteins only in the Htt-Q74 samples. All proteins migrated near their predicted molecular weights. For control, the TAPI procedure was conducted in parallel on the induced Htt-Q23 cell line (FUS, TDP-43, UBQLN2, HNRNPA1) or the un-induced Htt-Q74 cell line (CLINT1, HSPA8, RAD23B, SGTA). (C) Confocal microscopy shows localization of identified proteins to Htt-Q74 aggregates in PC12 cells. (left) RAD23B, nominally a DNA repair protein, localizes to nuclear Htt-Q74 inclusions but not cytoplasmic inclusions. (middle) FUS, an RNA-binding protein localizes to nuclear and cytoplasmic Htt-Q74 inclusions. (right) CLINT1, a clatherin-interacting protein, is observed in cytoplasmic Htt-Q74 aggregates. Arrows indicate foci with co-localized proteins. Green = GFP; Magenta = CLINT1, FUS or RAD23B in merge.