Figure 2.
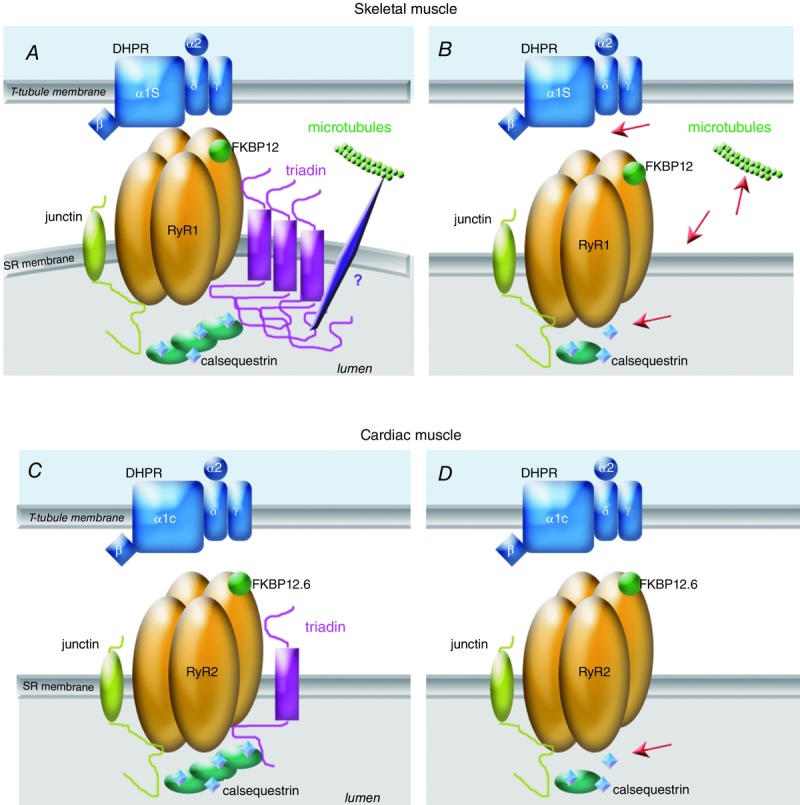
Function of triadin in skeletal and cardiac muscle, and alteration in triadin KO models A, in skeletal muscle of wild-type animals, the triadin isoform Trisk 95 forms polymers which induce membrane deformation, improving the DHPR–RyR contact and the EC coupling. Triadin anchors CSQ at the triad and via an indirect interaction allows the anchoring of the triad/CRC to the microtubules network. B, in the absence of triadin, the red arrows point to the major skeletal muscle defects: in the absence of membrane deformation, the EC coupling is altered, the amount of CSQ at the triad is reduced, the anchoring to the microtubules is altered, and some triads are not maintained in the normal transverse orientation. Association of the shorter skeletal muscle isoform Trisk 51 to Trisk 95 stops the elongation of these large Trisk 95 polymers. C, in cardiac muscle, the main function of the cardiac triadin Trisk 32 is the anchoring of CSQ in the vicinity of RyR2. D, this function is altered in triadin KO heart (red arrow). FKBP, FK506 binding protein.
