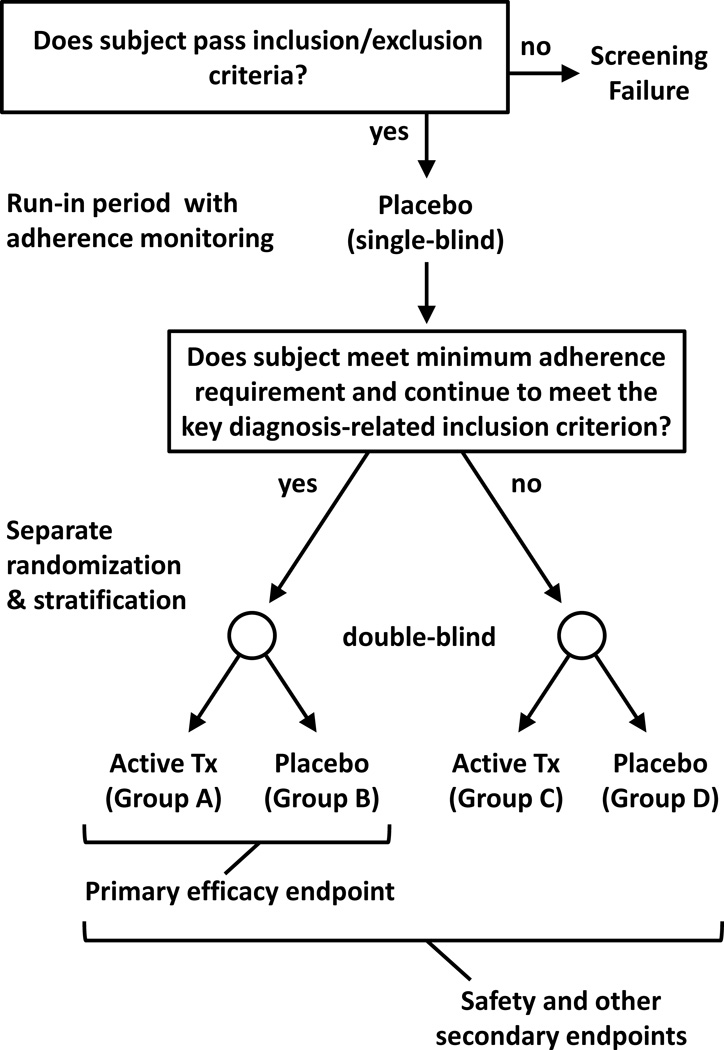
FIGURE 2.
Illustration of the RAMPUP study design. Subjects meeting study inclusion/exclusion criteria participate in a single-blind placebo (or adherence marker capsule) run-in period with adherence monitoring, followed by reassessment of a key diagnosis-related inclusion criterion (e.g., HAM-D score). Subjects who meet a specified minimum adherence requirement during the run-in period and continue to meet the inclusion criterion are prequalified for the primary efficacy endpoint (these subjects are randomized to Groups A & B in the illustration). An important feature of the study design is that all subjects continue to participate, even those who do not prequalify for the primary efficacy endpoint (these subjects are randomized to Groups C & D in the illustration). Data from all subjects are used for safety evaluations and other secondary endpoints.