Abstract
Despite a low risk of liver failure and preserved liver function, non-cirrhotic hepatocellular carcinoma (HCC) has a poor prognosis. In the current study, we evaluated an active regulator of SIRT1 (AROS) as a prognostic biomarker in non-cirrhotic HCC. mRNA levels of AROS were measured in tumor and non-tumor tissues obtained from 283 non-cirrhotic HCC patients. AROS expression was exclusively up-regulated in recurrent tissues from the non-cirrhotic HCC patients (P = 0.015) and also in tumor tissues irrespective of tumor stage (P < 0.001) or BCLC stage (P < 0.001). High mRNA levels of AROS were statistically significantly associated with tumor stage (P < 0.001), BCLC stage (P = 0.007), alpha fetoprotein (AFP) level (P = 0.013), microvascular invasion (P = 0.001), tumor size (P = 0.036), and portal vein invasion (P = 0.005). Kaplan-Meir curve analysis demonstrated that HCC patients with higher AROS levels had shorter disease-free survival (DFS) in both the short-term (P < 0.001) and long-term (P = 0.005) compared to those with low AROS. Cox regression analysis demonstrated that AROS is a significant predictor for DFS along with large tumor size, tumor multiplicity, vascular invasion, and poor tumor differentiation, which are the known prognostic factors. In conclusion, AROS is a significant biomarker for tumor aggressiveness in non-cirrhotic hepatocellular carcinoma.
Keywords: Carcinoma, Hepatocellular; Prognosis; Biological Markers
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most prevalent type of primary liver cancer and one of the most fatal solid tumors in the world (1). Most HCC are accompanied by liver cirrhosis, which is caused by multiple extrahepatic factors including alcohol, hepatitis viruses, and fatty liver disease (2,3,4,5). During the progression of cirrhosis, the liver undergoes fibrosis, scarring, and hepatocellular regeneration, resulting in the accumulation of aberrant cells with genetic mutations causally associated with liver malignancy (6,7).
Non-cirrhotic HCC comprises a relatively small proportion (10%-30%) of HCC cases (8). Since non-cirrhotic HCC has a low risk of liver failure, it is eligible for hepatic resection and has a generally good prognosis compared to cirrhotic HCC (9,10,11,12). In contrast, only a small proportion of cirrhotic HCC patients are amenable to hepatic resection. The pathology of non-cirrhotic HCC is peculiarly characterized by a large tumor size due to delayed diagnosis, resulting in a more aggressive clinical phenotype at the time of diagnosis. Additionally, fibrolamellar HCC (FL-HCC) is exclusively observed in noncirrhotics (7). Despite their prognostic importance and rare pathobiological attributes, little is known about the molecular characteristics of non-cirrhotic HCC compared to cirrhotic HCC. While cirrhosis plays certain roles to develop hepatocellular carcinoma in cirrhotic-HCC, in non-cirrhotic HCC, the molecular characteristics are more important for hepatocarcinogenesis in the absence of cirrhosis.
Active regulator of SIRT1 (AROS) is a ribosomal protein S19 (RPS19)-binding factor also known as RPS19BP1. In cancer, SIRT1 plays dual roles as a tumor promoter or suppressor through its extensive deacetylase activity toward many kinds of proteins such as p53, HSF1, FOXO3a, STAT3, and mTOR (13,14). In accordance with the multiple roles of SIRT1, its interacting partner AROS may have diverse functions in cancer through a SIRT1-dependent pathway. AROS is ubiquitously expressed in various mouse (15) and human organs but is overexpressed in cancer cell lines (16). Furthermore, AROS plays a role as an activator of SIRT1, which is frequently expressed at later stages of HCC (17,18). However, the expression of AROS and its clinical implications in HCC are not well explored and thus need to be investigated.
The aim of this study was to evaluate the underlying molecular characteristics of non-cirrhotic HCC. Specifically, we evaluated, for the first time, the expression of AROS and its clinical implications as a prognostic biomarker in non-cirrhotic HCC.
MATERIALS AND METHODS
Patients and tissues
HCC tissues and non-tumoral hepatic tissues were obtained with informed consent from 283 patients without cirrhosis who had undergone curative hepatectomy for primary HCC between 1995 and 2007 at Ajou University Medical Center, Samsung Medical Center, Keimyung University Dongsan Medical Center, and Hanyang University Medical Center in Korea. Tissue analysis and statistical analysis were performed at Cbs Bioscience Inc. The study protocol was approved by the respective Institutional Review Boards. All patients had adequate liver function reserve (class A=277, class B: 6) and had survived for at least 2 months after hepatectomy. Clinicopathological results and recurrence or death was obtained from the medical records of patients in each center and analyzed retrospectively. Non-cirrhotic HCC was defined by the lack of bridging fibrosis in background non-neoplastic liver tissues. We defined recurrence state as evidence of an overt new growing tumor mass in the remaining liver or as distant metastasis in radiologic studies including computed tomography or magnetic resonance imaging. In the current study, patients who had received treatment prior to surgery such as transcatheter arterial chemoembolization or radiofrequency ablation were excluded. Immediately after hepatectomy, fresh tumors and background livers were partly snap-frozen in liquid nitrogen and stored at -80℃ for RT-PCR analysis. The tumor grading followed the criteria proposed by Edmondson and Steiner (I, well differentiated; II, moderately differentiated; III, poorly differentiated; and IV, undifferentiated). The conventional tumor-node-metastasis system outlined in the Cancer Staging Manual (6th edition) published by the American Joint Committee on Cancer was used for tumor staging.
RNA extraction and cDNA synthesis
Total RNA was extracted from frozen HCC and non-tumoral hepatic tissues using an RNeasy minikit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Additionally, DNase I treatment was routinely included in the extraction step. The total RNA integrity was evaluated by a Bioanalyzer 2,100 (Agilent Technologies, Santa Clara, CA, USA). Four µg of total RNA was incubated with 2 µL of 10 µM oligo d (T)18 primer (Genotech, Daejeon, Korea) at 70℃ for 7 min and cooled on ice for 5 min. After adding the reverse transcriptase enzyme-containing master mix to the annealed total RNA sample, the reaction was incubated for 90 min at 42℃ followed by heat inactivation of the enzyme at 80℃ for 10 min. The cDNA samples were adjusted to a final volume of 400 µL by adding diethylpyrocarbonate (DEPC)-treated water.
Quantitative real-time PCR
Real-time PCR was performed as described previously (19). Using Applied Biosystems Prism 7900HT (Applied Biosystems, Foster City, CA, USA), real-time PCR was performed in a total volume of 10 µL with the amplification steps described below: an initial activation step at 95℃ for 10 min which was followed by 45 cycles of denaturation at 95℃ for 15 sec and elongation at 60℃ for 1 min. The primer and probe sequences were designed using Primer Express 3.0 software (Applied Biosystems), and all probes were labeled with FAM at the 5' end and with TAMRA at the 3' end. The sequence of primers and probes are displayed in Table S1. AROS was amplified and quantified using the forward primer, 5'-CGGAAGACGAAGGCAATTCA-3', the reverse primer 5'-GCCGACTTGGGCACCTTT-3', and the probe 5'-CC AGAAACTGCGGAACTCGGCCA-3'. The mRNA levels for AROS were measured (the threshold cycle, Ct) in triplicate and then normalized relative to a set of reference genes (beta-2-microglobulin [B2M], GAPDH, hydroxymethylbilane synthase [HMBS], hypoxanthine phosphoribosyltransferase 1 [HPRT1], and succinate dehydrogenase complex, subunit A, flavoprotein [SDHA]) with subtraction of the average expression for the five reference genes as an internal control (20). Using the difference between target gene Ct and average Ct of the reference genes (ΔCt values), the mRNA levels were calculated as 2-ΔCt.
Statistical analysis
Clinicopathologic characteristics of the HCC patients were evaluated using a chi square test and Fisher's exact test. The gene expression data were normalized by means of a log2 transform. After transformation, the results for each gene were centered and scaled to an average of 0 and SD of 1. Genes achieving P values of <0.05 in the Univariate Cox analyses were then input as potential predictors of patient risk. The classification accuracy was measured by the area under the curve (AUC) of the receiver-operator curves (ROC). Cumulative disease free survival (DFS) was analyzed using Kaplan-Meier survival curves. The statistical significance in different survival curves between the AROS-low group and the AROS-high group was examined by a log-rank test. Significant differences between gene expression levels for HCC and non-cancerous tissues were evaluated by a Student's t-test. A two-tailed P value test was used, with a P value of <0.05 considered statistically significant. All statistical analyses were conducted using the open source statistical programming environment R.
Ethics statements
This study protocol was approved by the institutional review board of Keimyung University Dongsan Medical Center (IRB No. 11-54), Ajou University Medical Center (AJIRB-GEN-KSP-09-278), Samsung Medical Center (2009-01-030-008), and Hanyang University Medical Center (HYG-09-11). Informed consent was waived by the board of each institution.
RESULTS
Clinicopathologic characteristics
A total of 283 patients were enrolled, 219 men and 64 women. The age ranged between 20 and 76 (mean of 52.0). The rates of hepatitis B and C were 63.6% (n=180) and 8.8% (n=25) respectively. Mean tumor size was 6.12 (range 1-20) cm and 68 patients (24.0%) had multiple tumors. The distribution of BCLC stages (A/B/C) were 45.2% (n=128), 42.0% (n=119) and 12.7% (n= 36), respectively. Patients at BCLC C stage consisted of 35 patients with portal vein invasion and 1 patient with impaired physical status (Table S2).
AROS is overexpressed in tumor tissues compared to non-tumor tissues in non-cirrhotic HCC
mRNA expression of AROS was examined in frozen tissues derived from non-cirrhotic HCC using real-time RT-PCR. mRNA levels of AROS were measured in triplicate and then normalized relative to the expression of 5 reference genes (B2M, GAPDH, HMBS, HPRT1, and SDHA) as an internal control.
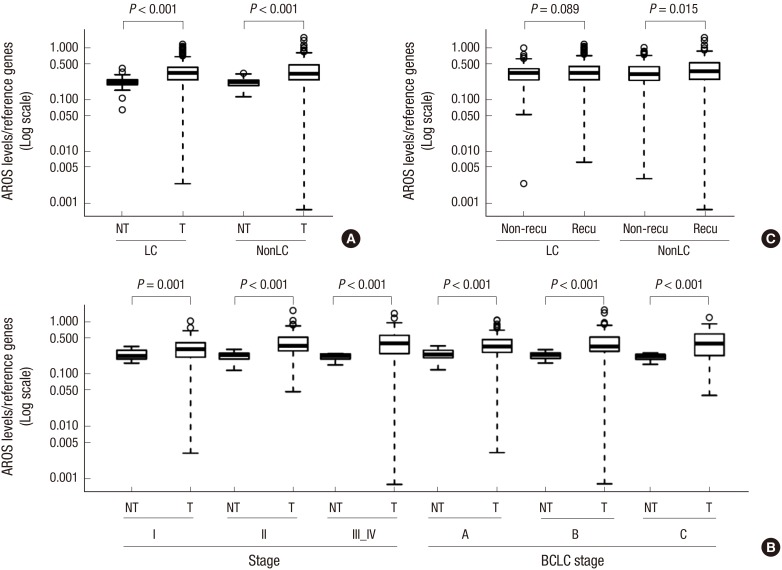
AROS mRNA was significantly higher in tumor tissues than in non-tumor tissues (0.2219 vs. 0.3706; mean copy number ratio, P<0.001; Fig. 1A). In non-cirrhotic HCC, AROS expression was significantly up-regulated in tumors compared to non-tumors at each tumor stage and its average expression in tumors increased along with the stages (stage I: 0.2331 vs. 0.3089; stage II: 0.2198 vs. 0.4021; stage III_IV: 0.2110 vs. 0.4187). Higher expression of AROS in tumors was also observed in all BCLC stages (stage A: 0.2313 vs. 0.3509; stage B: 0.2165 vs. 0.3830; stage C: 0.2036 vs. 0.3991) and differences between tumors and non-tumors were statistically significant (P<0.001; Fig. 1B).
Fig. 1. (A) AROS expression in HCC (T) compared to surrounding non-tumoral tissues (NT) in LC and NonLC. Both cirrhotic and non-cirrhotic tumors expressed significantly higher levels of AROS (P < 0.0001). (B) AROS expression in non-cirrhotic HCC (T) compared to surrounding non-tumoral tissues (NT) in total and across stages. mRNA levels of AROS were significantly up-regulated in tumors compared to non-tumors irrespective of tumor stages and BCLC stages. (C) Comparative analysis of AROS mRNA levels in HCC tissues with (recu) or without postoperative recurrence (non-recu) within 2 yr. AROS expression was higher in recurrent tumors than in non-recurrent tumors exclusively in NonLC, but not LC.
To examine the potential relevance of AROS expression with non-cirrhotic HCC prognosis, AROS expression was analyzed according to the presence of post-operative recurrence. mRNA levels of AROS were significantly higher in HCC patients that experienced recurrence within 2 yr of hepatic resection than in those who did not (0.3436 vs. 0.4039; mean copy number ratio, P=0.015; Fig. 1C). Furthermore, the gene expression pattern showing up-regulation of AROS in recurrent tissues remained significant in tissues at the 5-yr follow-up time point (0.3381 vs. 0.3981, P=0.014; Fig. S1).
Non-cirrhotic HCC patients with high expression of AROS have a shorter disease-free survival
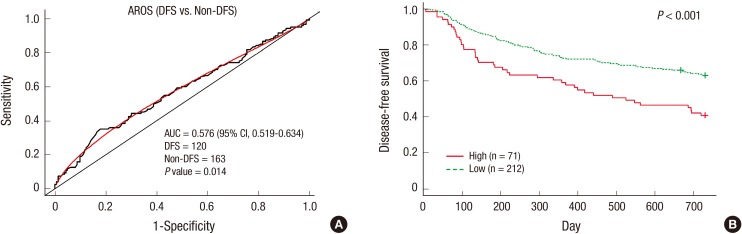
To assess the prognostic significance of AROS expression in the short term, ROC curves for DFS within 2 yr were constructed. The cutoff values for AROS mRNA levels (θ=1.075; 35.3% sensitivity, 82.2% specificity, and 62.2% accuracy) were determined based on the highest accuracy that discriminated between patients with good prognosis and poor prognosis from the ROC curves for DFS. The area under the curve (AUC) for the ROC curve showing the prediction of DFS within 2 yr by AROS mRNA level was 0.576 (Fig. 2A). The prognostic significance of AROS expression was further analyzed using the Kaplan-Meier method. At the 2-yr follow-up, 35.4% of the patients with low AROS expression experienced recurrence, whereas 57.8% of the patients with high AROS expression recurred (P<0.001). During 2 yr of follow-up, HCC patients with high levels of AROS displayed significantly shorter DFS time than those with low AROS levels (P<0.001; Fig. 2B).
Fig. 2. Non-cirrhotic HCC patients with high levels of AROS expression have a shorter DFS for 2 yr. Kaplan-Meier curves for the DFS of patients who showed high expression and low expression of AROS. (A) AUC value of AROS expression at prediction of DFS was 0.576. (B) The AROS-high group showed a significantly shorter DFS time than the AROS-low group for 2 yr (P < 0.001). Thin lines, patients expressed higher levels of AROS (n = 71); broken lines, patients expressed lower levels of AROS (n = 212).
Additionally, the long-term (5 yr) prognostic value of AROS was evaluated using the same cutoff value for AROS as for the 2-yr prognosis. The AUC value of the ROC curve used to stratify patients with shorter DFS by AROS levels was 0.575. The cutoff values for AROS mRNA level were the same as the values chosen for the short-term prognosis. The sensitivity, specificity, and accuracy were 30.2%, 81.5%, and 52.7%, respectively (Fig. S2A). At the time point of 5 yr, the KM plot indicated that non-cirrhotic HCC patients with high AROS mRNA levels had a significantly shorter DFS time (P=0.005; Fig. S2B).
However, in cirrhotic HCC, there was no significant difference in prognosis (DFS) between high and low AROS group (Fig. S3).
High mRNA levels of AROS are associated with tumor stage, BCLC stage, AFP level, vascular invasion, tumor size, and portal vein invasion in non-cirrhotic HCC
For a better understanding of the significance of AROS expression in non-cirrhotic HCC, the correlations between AROS expression and clinicopathological characteristics were analyzed by a chi-square and Fisher's exact tests (Table 1). According to the cutoff values of AROS levels determined by ROC curve analysis in Fig. 2, the patients were classified as two groups of AROS-high and AROS-low. High AROS expression was significantly correlated with tumor stage (P<0.001), BCLC stage (P=0.007), high α-fetoprotein (AFP) level (P=0.013), vascular invasion (P=0.001), large tumor size (P=0.036), and portal vein invasion (P=0.005). However, on multivariate analysis with binary logistic regression, there were no independent factors related to high AROS expression.
Table 1. Correlations of AROS mRNA with clinicopathologic characteristics.
| Clinicopathologic parameters | High AROS (n = 71) | Low AROS (n = 212) | P value |
|---|---|---|---|
| Age (< 55/ ≥ 55 yr) | 43/28 | 112/100 | 0.320 |
| Gender (Male/Female) | 53/18 | 166/46 | 0.624 |
| Hepatitis B infection (n, %) | 49 (69.1) | 131 (61.8) | 0.341 |
| Hepatitis C infection (-/+) | 4 (5.6%) | 21 (9.9%) | 0.392 |
| Tumor stage (I/II/III,IV) | 13/30/28 | 94/83/35 | < 0.001* |
| BCLC stage (A/B/C) | 24/31/16 | 104/88/20 | 0.007* |
| AFP level (> 100 ng/mL, %) | 41 (57.7) | 84 (39.6) | 0.013* |
| Microvascular invasion (n, %) | 57 (80.3) | 123 (58.0) | 0.001* |
| Tumor number (multiple, %)) | 26 (36.6) | 42 (19.8) | 0.007* |
| Tumor size (> 5 cm, %) | 43 (60.6) | 96 (45.3) | 0.036* |
| Edmondson grade (I/II/III,IV) | 3/60/83 | 22/152/38 | 0.087 |
| Portal Vein Invasion (n, %) | 16 (22.3) | 19 (9.0) | 0.005* |
*Analyzed by chi square test. BCLC, Barcelona Clinic Liver Cancer; AFP, alpha fetoprotein.
mRNA level of AROS is associated with both short- and long-term prognosis in non-cirrhotic HCC
A univariate Cox regression analysis was used to identify important prognostic factors for DFS (Table 2). For the non-cirrhotic HCCs analyzed by RT-PCR, a high AROS mRNA level was identified as one of the important risk factors for 2-yr DFS. In the same manner, the prognostic significance of AROS levels and clinicopathological variables was further evaluated for long-term (5-yr) DFS. Higher expression of AROS mRNA (P=0.005) was also significantly associated with 5 yr DFS. However, in multivariate analysis, AROS mRNA level was not a significant risk factor (P=0.080; Table 2, Table S3).
Table 2. Univariate and multivariate analysis of prognostic factors for 2-yr disease-free survival.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (< 55 yr/ ≥ 55 yr) | 1.11 | 0.77-1.59 | 0.574 | |||
| Gender (male/female) | 1.44 | 0.96-2.14 | 0.077 | |||
| Edmondson grade (I, II vs. III, IV) | 1.75 | 1.13-2.70 | 0.012 | |||
| HBV (absent vs. present) | 1.33 | 0.91-1.95 | 0.145 | |||
| HCV (absent vs. present) | 1.46 | 0.84-2.56 | 0.181 | |||
| AFP level (< 100 ng/mL vs. ≥ 100 ng/mL) | 1.91 | 1.33-2.74 | 0.000 | |||
| Tumor size (≤ 5 cm vs. > 5 cm) | 2.37 | 1.64-3.44 | 0.000 | |||
| Tumor stage (I, II vs. III, IV) | 4.38 | 3.02-6.34 | 0.000 | 2.26 | 1.17-4.36 | 0.01 |
| Vascular invasion (absent vs. present) | 3.83 | 2.37-6.20 | 0.000 | 2.27 | 1.33-3.87 | 0.01 |
| Tumor number (single vs. multi) | 3.06 | 2.12-4.43 | 0.000 | |||
| AROS (low vs. high) | 1.95 | 1.34-2.83 | 0.001 | 1.42 | 0.95-2.12 | 0.08 |
HBV, hepatitis B; HCV, hepatitis C; AFP, alpha fetoprotein.
DISCUSSION
Non-cirrhotic HCC is well known to have specific macroscopic features including a single and large tumor mass due to both delayed diagnosis and a lack of research on screening and prognostic factors (3,8,21,22). We consistently observed a considerably higher proportion of single tumors than multiple tumors (Table 2). Additionally, patients with tumor sizes over 5 cm comprised about half of all patients. These versatile characteristics of non-cirrhotic HCC often worsen the prognosis for non-cirrhotics and increase the difficulty of treating non-cirrhotic HCC. In the current study, a specific biomarker, AROS (RPS19BP1), was identified for the prediction of non-cirrhotic HCC prognosis.
Using high-throughput gene expression analysis, we investigated differentially expressed genes in non-cirrhotic HCC tissues. We found AROS to be a unique and significant prognostic biomarker for non-cirrhotic HCC, not cirrhosis (data not shown). Comparative analysis of AROS mRNA expression between tumor and non-tumoral tissues revealed that AROS is highly expressed in tumors compared to non-tumors (Fig. 1A) and interestingly, AROS was significantly overexpressed in recurrent tumors compared to non-recurrent ones (Fig. 1B). Stage analysis also disclosed higher levels of AROS in tumors across all tumor stages and BCLC stages. Additionally, AROS expression increased with advanced tumor stages, and vascular invasion (Fig. 1C). Although AROS expression has not been studied previously in tumor versus non-tumor tissues of patients, there is evidence supporting our results. AROS is ubiquitously expressed in various mouse (15) and human organs but is overexpressed in cancer cell lines (16). Since AROS regulates ribosome biogenesis, changes to the gene expression for AROS and the resulting reduction in ribosome function may contribute to tumor progression (18,23,24,25). In agreement with our speculation, dysregulation of ribosome biogenesis and translation was found to be associated with breast cancer progression (25). Furthermore, the role of AROS as an activator for SIRT1, which is frequently expressed at later stages of HCC, has been reported (17,18). In addition, we confirmed that AROS is a significant poor prognostic factor for both the short-term and long-term in non-cirrhotic HCC (Fig. 2 and Fig. S2). Although we did not investigate correlations between AROS and SIRT1 in the current study, these results imply that AROS may be related to tumor progression in a SIRT1-dependent manner. In agreement with our supposition, significant overexpression of SIRT1 was observed in HCC patients with shorter postoperative survival (26,27). Collectively, our findings suggest that AROS could be associated with the tumor aggressiveness of HCC. To our knowledge, we demonstrated for the first time that AROS is overexpressed in non-cirrhotic HCC, suggesting the potential of AROS as a prognostic biomarker.
In cirrhotic livers, the impact of AROS on carcinogenesis may be less than other factors, including the presence of cirrhosis. In non-cirrhotic HCC, however, AROS has an important role in tumor progression and recurrence. Short-term recurrence and long-term recurrence have been considered to have different origins and recurrence mechanisms. Considering that short-term recurrence and long-term recurrence could be affected by the primary tumor (unicentric origin) and by surrounding tissue predisposed to neoplastic transformation (multicentric origin), AROS may affect both processes in non-cirrhotic HCC. In this regard, for improved clinical outcomes of the curative-intent treatment in non-cirrhotic HCC, identification of prognostic factors accompanied by new treatment strategies are needed. For instance, non-cirrhotic HCC patients with a high expression of AROS should be monitored for the short term and could be considered for salvage transplantation after surgery. The information related to AROS expression in non-cirrhotic HCC would be important not only for evaluating the mechanism for carcinogenesis, but also for improving patients' survival in particular. If our findings can be reproduced in a larger cohort, they can potentially provide benefits for predicting the prognosis and therapeutic strategies to eventually improve the outcome of non-cirrhotic HCC.
Viral infection can trigger hepatic carcinogenesis independently of the development of cirrhosis (7,28). Therefore, in patients with viral hepatitis, the development of HCC would be influenced by the presence of viral hepatitis as well as cirrhosis. In the current cohort, HBV-positive HCC was dominant in cirrhotic HCC whereas HCV was frequently observed in non-cirrhotic HCC (Table S2). These results are different from those of a previous comparative analysis between non-cirrhotic and cirrhotic HCC in a western institution demonstrating that hepatitis C was dominant in cirrhotic HCC (29). However, the incidences of hepatitis B or C were variable in several studies (7). The molecular characteristics would vary according to viral status. Regarding AROS expression, we did not observe a significant association with the viral status. Further study of the association between viral status and AROS expression and validation in other cohorts is necessary. However, our results also had some drawbacks. Multivariate Cox analysis did not demonstrate the significance of AROS as an independent prognostic factor. Considering that 44.4% of AROS-high patients are in advanced stages (tumor stage III-IV and BCLC C stage), the AROS-high group might show poor prognosis in a stage-dependent manner. Although AROS expression is not an independent prognostic factor, it is strongly related with tumor aggressiveness such as advanced stage and poor differentiation. Therefore, AROS warrants further investigation as a therapeutic target in advanced non-cirrhotic HCC. In conclusion, AROS is a significant biomarker for tumor aggressiveness in non-cirrhotic HCC.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to Hyein Kang and Yeokyung Nam (BS, Keimyung University School of Medicine) for assistance in data collection.
Footnotes
This research was supported by the annex research institute (Medical Genetics Institute) Research Grant of Keimyung University in 2011, the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (Grant number: NRF-2013R1A1A4A01009053) and the National R&D Program for Cancer Control, Ministry of Health & Welfare (1320240).
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Manuscript writing: Kwon JH, Ahn KS, Kang KJ. Experimental design: Kwon JH, Moon YH, Park JY, Joh JW, Kang KJ, Lee KG. Experiment: Kwon JH. Data analysis: Moon YH, Park JY, Wang HJ. Manuscript editing: Ahn KS, Choi KY, Kim G, Kang KJ, Joh JW.
Supplementary Material
Table S1. Sequences of primers and probes.
| Genes | Sequences | |
|---|---|---|
| AROS | F | CGGAAGACGAAGGCAATTCA |
| R | GCCGACTTGGGCACCTTT | |
| P | CCAGAAACTGCGGAACTCGGCCA | |
| B2M | F | CATTCGGGCCGAGATGTCT |
| R | CTCCAGGCCAGAAAGAGAGAGTAG | |
| P | CCGTGGCCTTAGCTGTGCTCGC | |
| GAPDH | F | CACATGGCCTCCAAGGAGTAA |
| R | TGAGGGTCTCTCTCTTCCTCTTGT | |
| P | CTGGACCACCAGCCCCAGCAAG | |
| HMBS | F | CCAGGGATTTGCCTCACCTT |
| R | AAAGAGATGAAGCCCCCACAT | |
| P | CCTTGATGACTGCCTTGCCTCCTCAG | |
| HPRT1 | F | GCTCGAGATGTGATGAAGGAGAT |
| R | CCAGCAGGTCAGCAAAGAATT | |
| P | CCATCACATTGTAGCCCTCTGTGTGCTC | |
| SDHA | F | CACCTAGTGGCTGGGAGCTT |
| R | GCCCAGTTTTATCATCTCACAAGA | |
| P | TGGCACTTACCTTTGTCCCTTGCTTCA |
Table S2. Comparison of clinicopathological information between cirrhotic HCC and non-cirrhotic HCC.
| Clinicopathologic parameters | Cirrhotic HCC (n = 249) | Non-cirrhotic HCC (n = 283) | P value |
|---|---|---|---|
| Age (< 55 / ≥ 55 yr) | 150/99 | 155/128 | 0.236 |
| Gender (Male/Female) | 198/51 | 219/64 | 0.624 |
| Hepatitis B infection (n, %) | 221 (88.9) | 180 (63.6) | < 0.001* |
| Hepatitis C infection (n, %) | 10 (4.0) | 25 (8.8) | 0.042* |
| Tumor stage (I/II/III, IV) | 105/96/48 | 107/113/63 | 0.532 |
| BCLC stage (A/B/C) | 142/75/32 | 128/119/36 | 0.012* |
| AFP level (> 100 ng/mL, %) | 125 (50.2) | 125 (44.1) | 0.205 |
| Microvascular invasion (n, %) | 141 (56.6) | 180 (63.6) | 0.109 |
| Tumor number (multiple, %) | 58 (23.3) | 68 (24.0) | 0.923 |
| Tumor size (≤ 5 / > 5 cm) | 175/74 | 144/139 | < 0.001* |
| Edmondson grade (I/II/III, IV) | 27/169/53 | 25/21/46 | 0.195 |
| Portal Vein Invasion (n, %) | 32 (12.8) | 35 (12.3) | 0.971 |
*Analyzed by chi square test. BCLC, Barcelona Clinic Liver Cancer; AFP, alpha fetoprotein.
Table S3. Univariate and multivariate analysis of prognostic factors for 5-yr disease-free survival.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (< 55 yr / ≥ 55 yr) | 1.07 | 0.78-1.46 | 0.662 | |||
| Gender (male/female) | 1.55 | 1.10-2.19 | 0.012 | |||
| Edmondson grade (I, II vs. III, IV) | 1.71 | 1.15-2.53 | 0.078 | |||
| HBV (absent vs. present) | 1.29 | 0.93-1.79 | 0.131 | |||
| HCV (absent vs. present) | 1.54 | 0.94-1.42 | 0.085 | |||
| AFP level (< 100 ng/mL vs. ≥ 100 ng/mL) | 1.63 | 1.19-2.23 | 0.002 | |||
| Tumor size (≤ 5 cm vs. > 5 cm) | 1.94 | 1.42-2.66 | 0.000 | |||
| Tumor stage (I, II vs. III, IV) | 3.36 | 2.40-4.71 | 0.000 | |||
| Vascular Invasion (absent vs. present) | 2.92 | 2.01-4.24 | 0.000 | 2.13 | 1.40-3.24 | 0.001 |
| Tumor number (single vs. multi) | 2.61 | 1.87-3.64 | 0.000 | |||
| AROS (low vs. high) | 1.61 | 1.15-2.27 | 0.005 | 1.21 | 0.84-1.73 | 0.309 |
Comparative analysis of AROS mRNA levels in HCC tissues with (recu) or without postoperative recurrence (non-recu) within 5 yr. AROS expression was higher in recurrent tumors than in non-recurrent tumors exclusively in NonLC.
Non-cirrhotic HCC patients with high levels of AROS expression have a shorter DFS for 5 yr. (A) AUC was 0.575. (B) AROS-high group showed significant shorter DFS time (P = 0.00495). Thin lines, patients expressed higher levels of AROS (n = 71); broken lines, patients expressed lower levels of AROS (n = 212).
Cirrhotic HCC showed no significant difference in prognosis (DFS) between AROS-high and AROS-low groups.
References
- 1.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kew MC, Popper H. Relationship between hepatocellular carcinoma and cirrhosis. Semin Liver Dis. 1984;4:136–146. doi: 10.1055/s-2008-1040653. [DOI] [PubMed] [Google Scholar]
- 3.Trevisani F, D'Intino PE, Caraceni P, Pizzo M, Stefanini GF, Mazziotti A, Grazi GL, Gozzetti G, Gasbarrini G, Bernardi M. Etiologic factors and clinical presentation of hepatocellular carcinoma. Differences between cirrhotic and noncirrhotic Italian patients. Cancer. 1995;75:2220–2232. doi: 10.1002/1097-0142(19950501)75:9<2220::aid-cncr2820750906>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Nzeako UC, Goodman ZD, Ishak KG. Hepatocellular carcinoma in cirrhotic and noncirrhotic livers. A clinico-histopathologic study of 804 North American patients. Am J Clin Pathol. 1996;105:65–75. doi: 10.1093/ajcp/105.1.65. [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 6.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42:341–347. doi: 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Huang L, Li BK, Zhang YQ, Li JQ, Yuan YF. Clinicopathologic features and long-term outcomes of Chinese patients with hepatocellular carcinoma in non-cirrhotic liver. Dig Surg. 2008;25:376–382. doi: 10.1159/000170881. [DOI] [PubMed] [Google Scholar]
- 9.Laurent C, Blanc JF, Nobili S, Sa Cunha A, le Bail B, Bioulac-Sage P, Balabaud C, Capdepont M, Saric J. Prognostic factors and longterm survival after hepatic resection for hepatocellular carcinoma originating from noncirrhotic liver. J Am Coll Surg. 2005;201:656–662. doi: 10.1016/j.jamcollsurg.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Bismuth H, Chiche L, Castaing D. Surgical treatment of hepatocellular carcinomas in noncirrhotic liver: experience with 68 liver resections. World J Surg. 1995;19:35–41. doi: 10.1007/BF00316977. [DOI] [PubMed] [Google Scholar]
- 11.Nagasue N, Ono T, Yamanoi A, Kohno H, El-Assal ON, Taniura H, Uchida M. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg. 2001;88:515–522. doi: 10.1046/j.1365-2168.2001.01732.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen MF, Tsai HP, Jeng LB, Lee WC, Yeh CN, Yu MC, Hung CM. Prognostic factors after resection for hepatocellular carcinoma in noncirrhotic livers: univariate and multivariate analysis. World J Surg. 2003;27:443–447. doi: 10.1007/s00268-002-6708-7. [DOI] [PubMed] [Google Scholar]
- 13.Kim EJ, Um SJ. SIRT1: roles in aging and cancer. BMB Rep. 2008;41:751–756. doi: 10.5483/bmbrep.2008.41.11.751. [DOI] [PubMed] [Google Scholar]
- 14.Song NY, Surh YJ. Janus-faced role of SIRT1 in tumorigenesis. Ann N Y Acad Sci. 2012;1271:10–19. doi: 10.1111/j.1749-6632.2012.06762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda N, Toku S, Kenmochi N, Tanaka T. A novel nucleolar protein interacts with ribosomal protein S19. Biochem Biophys Res Commun. 2006;339:41–46. doi: 10.1016/j.bbrc.2005.10.184. [DOI] [PubMed] [Google Scholar]
- 16.Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28:277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Knight JR, Allison SJ, Milner J. Active regulator of SIRT1 is required for cancer cell survival but not for SIRT1 activity. Open Biol. 2013;3:130130. doi: 10.1098/rsob.130130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knight JR, Willis AE, Milner J. Active regulator of SIRT1 is required for ribosome biogenesis and function. Nucleic Acids Res. 2013;41:4185–4197. doi: 10.1093/nar/gkt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon JH, Kim J, Park JY, Hong SM, Park CW, Hong SJ, Park SY, Choi YJ, Do IG, Joh JW, et al. Overexpression of high-mobility group box 2 is associated with tumor aggressiveness and prognosis of hepatocellular carcinoma. Clin Cancer Res. 2010;16:5511–5521. doi: 10.1158/1078-0432.CCR-10-0825. [DOI] [PubMed] [Google Scholar]
- 20.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smalley SR, Moertel CG, Hilton JF, Weiland LH, Weiand HS, Adson MA, Melton LJ, 3rd, Batts K. Hepatoma in the noncirrhotic liver. Cancer. 1988;62:1414–1424. doi: 10.1002/1097-0142(19881001)62:7<1414::aid-cncr2820620729>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Capussotti L, Muratore A, Amisano M, Massucco P, Polastri R, Bouzari H. Liver resection for large-size hepatocellular carcinomas in 47 non-cirrhotic patients--no mortality and long-term survival. Hepatogastroenterology. 2006;53:768–772. [PubMed] [Google Scholar]
- 23.Donati G, Montanaro L, Derenzini M. Ribosome biogenesis and control of cell proliferation: p53 is not alone. Cancer Res. 2012;72:1602–1607. doi: 10.1158/0008-5472.CAN-11-3992. [DOI] [PubMed] [Google Scholar]
- 24.Deisenroth C, Zhang Y. Ribosome biogenesis surveillance: probing the ribosomal protein-Mdm2-p53 pathway. Oncogene. 2010;29:4253–4260. doi: 10.1038/onc.2010.189. [DOI] [PubMed] [Google Scholar]
- 25.Belin S, Beghin A, Solano-Gonzalez E, Bezin L, Brunet-Manquat S, Textoris J, Prats AC, Mertani HC, Dumontet C, Diaz JJ. Dysregulation of ribosome biogenesis and translational capacity is associated with tumor progression of human breast cancer cells. PLoS One. 2009;4:e7147. doi: 10.1371/journal.pone.0007147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen HC, Jeng YM, Yuan RH, Hsu HC, Chen YL. SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann Surg Oncol. 2012;19:2011–2019. doi: 10.1245/s10434-011-2159-4. [DOI] [PubMed] [Google Scholar]
- 27.Jang KY, Noh SJ, Lehwald N, Tao GZ, Bellovin DI, Park HS, Moon WS, Felsher DW, Sylvester KG. SIRT1 and c-Myc promote liver tumor cell survival and predict poor survival of human hepatocellular carcinomas. PLoS One. 2012;7:e45119. doi: 10.1371/journal.pone.0045119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 29.Beard RE, Hanto DW, Gautam S, Miksad RA. A comparison of surgical outcomes for noncirrhotic and cirrhotic hepatocellular carcinoma patients in a Western institution. Surgery. 2013;154:545–555. doi: 10.1016/j.surg.2013.02.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sequences of primers and probes.
| Genes | Sequences | |
|---|---|---|
| AROS | F | CGGAAGACGAAGGCAATTCA |
| R | GCCGACTTGGGCACCTTT | |
| P | CCAGAAACTGCGGAACTCGGCCA | |
| B2M | F | CATTCGGGCCGAGATGTCT |
| R | CTCCAGGCCAGAAAGAGAGAGTAG | |
| P | CCGTGGCCTTAGCTGTGCTCGC | |
| GAPDH | F | CACATGGCCTCCAAGGAGTAA |
| R | TGAGGGTCTCTCTCTTCCTCTTGT | |
| P | CTGGACCACCAGCCCCAGCAAG | |
| HMBS | F | CCAGGGATTTGCCTCACCTT |
| R | AAAGAGATGAAGCCCCCACAT | |
| P | CCTTGATGACTGCCTTGCCTCCTCAG | |
| HPRT1 | F | GCTCGAGATGTGATGAAGGAGAT |
| R | CCAGCAGGTCAGCAAAGAATT | |
| P | CCATCACATTGTAGCCCTCTGTGTGCTC | |
| SDHA | F | CACCTAGTGGCTGGGAGCTT |
| R | GCCCAGTTTTATCATCTCACAAGA | |
| P | TGGCACTTACCTTTGTCCCTTGCTTCA |
Table S2. Comparison of clinicopathological information between cirrhotic HCC and non-cirrhotic HCC.
| Clinicopathologic parameters | Cirrhotic HCC (n = 249) | Non-cirrhotic HCC (n = 283) | P value |
|---|---|---|---|
| Age (< 55 / ≥ 55 yr) | 150/99 | 155/128 | 0.236 |
| Gender (Male/Female) | 198/51 | 219/64 | 0.624 |
| Hepatitis B infection (n, %) | 221 (88.9) | 180 (63.6) | < 0.001* |
| Hepatitis C infection (n, %) | 10 (4.0) | 25 (8.8) | 0.042* |
| Tumor stage (I/II/III, IV) | 105/96/48 | 107/113/63 | 0.532 |
| BCLC stage (A/B/C) | 142/75/32 | 128/119/36 | 0.012* |
| AFP level (> 100 ng/mL, %) | 125 (50.2) | 125 (44.1) | 0.205 |
| Microvascular invasion (n, %) | 141 (56.6) | 180 (63.6) | 0.109 |
| Tumor number (multiple, %) | 58 (23.3) | 68 (24.0) | 0.923 |
| Tumor size (≤ 5 / > 5 cm) | 175/74 | 144/139 | < 0.001* |
| Edmondson grade (I/II/III, IV) | 27/169/53 | 25/21/46 | 0.195 |
| Portal Vein Invasion (n, %) | 32 (12.8) | 35 (12.3) | 0.971 |
*Analyzed by chi square test. BCLC, Barcelona Clinic Liver Cancer; AFP, alpha fetoprotein.
Table S3. Univariate and multivariate analysis of prognostic factors for 5-yr disease-free survival.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (< 55 yr / ≥ 55 yr) | 1.07 | 0.78-1.46 | 0.662 | |||
| Gender (male/female) | 1.55 | 1.10-2.19 | 0.012 | |||
| Edmondson grade (I, II vs. III, IV) | 1.71 | 1.15-2.53 | 0.078 | |||
| HBV (absent vs. present) | 1.29 | 0.93-1.79 | 0.131 | |||
| HCV (absent vs. present) | 1.54 | 0.94-1.42 | 0.085 | |||
| AFP level (< 100 ng/mL vs. ≥ 100 ng/mL) | 1.63 | 1.19-2.23 | 0.002 | |||
| Tumor size (≤ 5 cm vs. > 5 cm) | 1.94 | 1.42-2.66 | 0.000 | |||
| Tumor stage (I, II vs. III, IV) | 3.36 | 2.40-4.71 | 0.000 | |||
| Vascular Invasion (absent vs. present) | 2.92 | 2.01-4.24 | 0.000 | 2.13 | 1.40-3.24 | 0.001 |
| Tumor number (single vs. multi) | 2.61 | 1.87-3.64 | 0.000 | |||
| AROS (low vs. high) | 1.61 | 1.15-2.27 | 0.005 | 1.21 | 0.84-1.73 | 0.309 |
Comparative analysis of AROS mRNA levels in HCC tissues with (recu) or without postoperative recurrence (non-recu) within 5 yr. AROS expression was higher in recurrent tumors than in non-recurrent tumors exclusively in NonLC.
Non-cirrhotic HCC patients with high levels of AROS expression have a shorter DFS for 5 yr. (A) AUC was 0.575. (B) AROS-high group showed significant shorter DFS time (P = 0.00495). Thin lines, patients expressed higher levels of AROS (n = 71); broken lines, patients expressed lower levels of AROS (n = 212).
Cirrhotic HCC showed no significant difference in prognosis (DFS) between AROS-high and AROS-low groups.