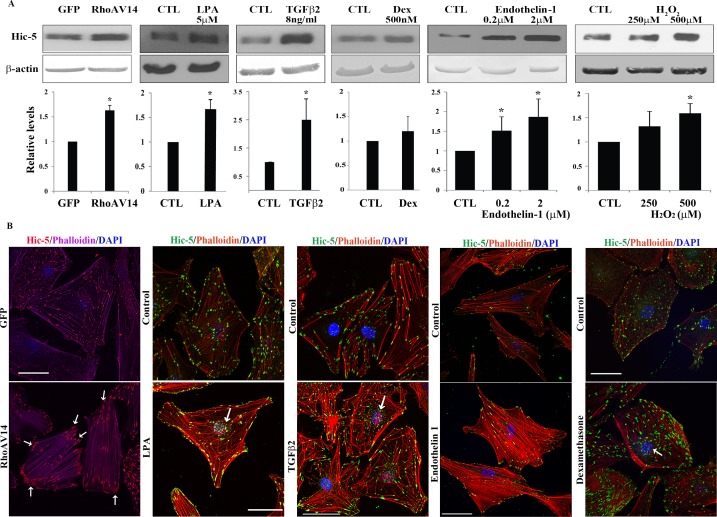
Figure 2.
Upregulation of expression and redistribution of Hic-5 in human TM cells by various physiological factors and Rho GTPase. (A) Confluent serum-starved HTM cells expressing either adenovirus-encoded RhoAV14 or GFP, or treated with LPA (5 μM for 24 hours), TGF-β2 (8 ng/mL for 14 hours), dexamethasone (500 nM for 4 days), endothelin-1 (0.2 and 2 μM for 24 hours), or H2O2 (250 and 500 μM for 3 hours), exhibit a significant increase in Hic-5 protein levels except with dexamethasone relative to untreated control cells, as assessed by immunoblotting and densitometric quantification. β-actin was immunoblotted as a loading control (n = 6 for dexamethasone; for all other analyses n = 4, mean ± SD). *P < 0.05. (B) HTM cells expressing RhoAV14/GFP or GFP and wild-type cells were grown to semiconfluence on gelatin-coated glass coverslips. The wild-type cells were treated with LPA, TGF-β2, endothelin-1 (2 μM), or dexamethasone (500 nM) as described above. All cells were serum starved prior to being immunostained for Hic-5 and labeled for F-actin (using rhodamine-phalloidin) followed by confocal microscopy imaging to evaluate changes in Hic-5 distribution and actin cytoskeletal reorganization. Expression of RhoAV14/GFP or treatment of HTM cells with LPA, TGF-β2, and endothelin-1 led to appearance of a contractile cell morphology with increased actin stress fibers (in cells either expressing RhoAV14 or treated with endothelin-1) and tangled or cross-linked actin (cells treated with LPA, TGF-β2, or dexamethasone). Hic-5 redistributed to the leading ends of actin stress fibers (in cells either expressing RhoAV14 or treated with endothelin-1) and tangled actin (cells treated with LPA, TGF-β2, or dexamethasone) relative to GFP-expressing controls or untreated controls. Also seen is increased localization of Hic-5 to the nucleus or nuclear matrix (arrows) in cells treated with LPA, TGF-β2, and dexamethasone. Scale bars: 50 μm.