Figure 3.
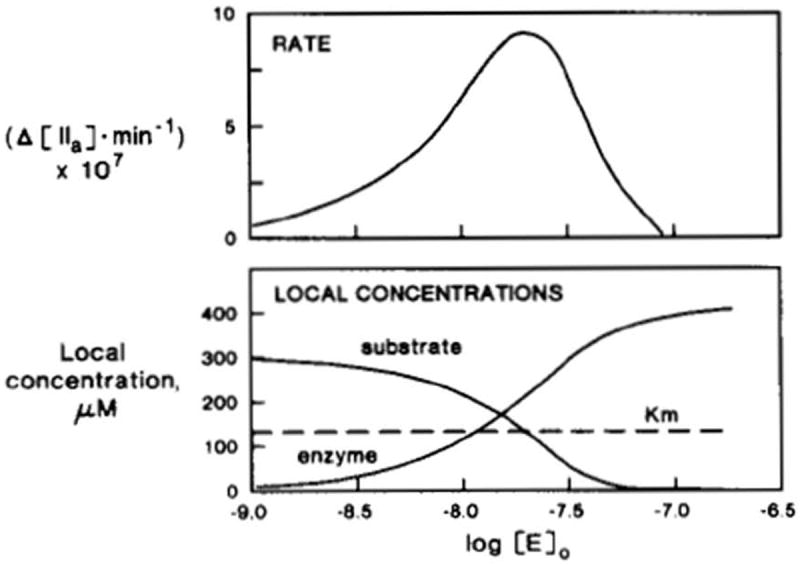
Simulated inhibition of initial rates of prothrombin activation by excess enzyme [39]. The logarithms of enzyme concentrations are indicated by the units of the horizontal axis. Top, simulated reaction rates (moles of thrombin*min-1) are shown; bottom, local (interface shell) concentrations are shown for both substrate and enzyme. With increased levels of added enzyme, its local concentration increases, resulting in increased reaction rates. Excess enzyme, however, competes with the substrate in the interface shell, lowering its local concentration, thus resulting in decreased reaction rates. This research was originally published in J Biol Chem. Nesheim ME, Tracy RP, Mann KG. “Clotspeed,” a mathematical simulation of the functional properties of prothrombinase. J Biol Chem. 1984; 259: 1447-53. © The American Society for Biochemistry and Molecular Biology.
