Abstract
Based on the mechanism of pathophysiology, thalassemia major or transfusion-dependent thalassemia patients may have an increased risk of developing organic erectile dysfunction resulting from hypogonadism. However, there have been few studies investigating the association between erectile dysfunction and transfusion-naive thalassemia populations. We constructed a population-based cohort study to elucidate the association between transfusion-naive thalassemia populations and organic erectile dysfunction
This nationwide population-based cohort study involved analyzing data from 1998 to 2010 obtained from the Taiwanese National Health Insurance Research Database, with a follow-up period extending to the end of 2011. We identified men with transfusion-naive thalassemia and selected a comparison cohort that was frequency-matched with these according to age, and year of diagnosis thalassemia at a ratio of 1 thalassemia man to 4 control men. We analyzed the risks for transfusion-naive thalassemia men and organic erectile dysfunction by using Cox proportional hazards regression models.
In this study, 588 transfusion-naive thalassemia men and 2337 controls were included. Total 12 patients were identified within the thalassaemia group and 10 within the control group. The overall risks for developing organic erectile dysfunction were 4.56-fold in patients with transfusion-naive thalassemia men compared with the comparison cohort after we adjusted for age and comorbidities.
Our long-term cohort study results showed that in transfusion-naive thalassemia men, there was a higher risk for the development of organic erectile dysfunction, particularly in those patients with comorbidities.
INTRODUCTION
Male sexual dysfunction can be classified as erectile dysfunction (ED), diminished sexual desire disorders, or abnormal ejaculation. ED, defined as difficulty in achieving or maintaining an erection during sexual intercourse, is the most common form of sexual dysfunction in adult men.1 The risk factors for ED are complex and can generally be classified into 2 distinct groups, namely systemic organic and psychological causes.2 The major risk factors resulting in organic ED include chronic cardiovascular and metabolic causes such as diabetes, coronary artery disease, hypertension, stroke, and hyperlipidemia.3 In a previous study, 26.5% of patients with ED were anemic. Therefore, anemia of any cause may contribute to ED.4 In addition, age is an independent risk factor for ED, and the prevalence of complete ED increases with age. In particular, approximately 30% to 40% of men in their 80s have complete ED.5,6
Thalassemia is an autosomal recessive hereditary hemoglobinopathy that manifests as microcytic hypochromic anemia and has varied clinical presentations ranging from asymptomatic to severe lethal complications caused by hemolytic anemia, ineffective hematopoiesis, or transfusion dependence. Although thalassemia has various clinically distinct phenotypes and complex genotypes, transfusion dependence may be considered as a marker of severity to distinguish between thalassemia major and other types of thalassemia. Recently, thalassemia has been classified into 3 distinct groups according to clinical characteristics and transfusion dependence as follows: asymptomatic thalassemia trait/minor, nontransfusion-dependent thalassemia (NTDT), and thalassemia major.7 Patients with α/β thalassemia major usually require repeated blood transfusions because of ineffective hematopoiesis and severe hemolytic anemia. Secondary iron overloading frequently results in target-organ toxicity such as heart failure, osteoporosis, or hypogonadism.8
In patients with thalassemia major or transfusion-dependent thalassemia, ED is a possible complication predominantly due to iron overload as a result of recurrent blood transfusion leading to hypogonadism.9 However, most thalassemia populations are usually transfusion-naive and asymptomatic, with much fewer having NTDT syndromes or thalassemia major. Although these patients have less severe clinical manifestations and have not received blood transfusions, they may also have a risk of developing ED via the pathophysiological mechanisms of anemia and chronic hemolysis. However, studies investigating the relationships between these groups and the incidence of ED are rare. The purpose of this nationwide population-based cohort study was to clarify the relationship between transfusion-naive thalassemia populations and ED in a multiethnic male population.
METHODS
Data Source
The National Health Insurance (NHI) Program was implemented on March 1, 1995, and this program covers >99% of the 23.72 million people in Taiwan. The Longitudinal Health Insurance Database 2000 (LHID 2000), which was released by the National Health Research Institutes (NHRI), was used in this retrospective cohort study. The NHRI established a National Health Insurance Research Database (NHIRD) to record the medical services of all beneficiaries, including inpatient and outpatient demographics, primary and secondary diagnoses, procedures, prescriptions, and medical expenditures. The LHID 2000 includes 1 million insurants randomly selected from the 2000 Registry for Beneficiaries, which contains all medical records of each insurant from 1996 to 2011. The LHID 2000 was released by the NHRI, of which the institute claims detailed examinations of each International Classification of Diseases, Ninth Revision (ICD-9-CM) coding. The NHRI has also reported no significant difference in age, or health care costs between cohorts in the LHID 2000 and all insurance enrollees. 10 Disease was identified based on the ICD-9-CM codes registered in the LHID 2000. We used secondary data with deidentified analysis; therefore, no informed consent was required. This study was approved by the Ethics Review Board of China Medical University (CMU-REC-101-012).
Sampled Participants
We selected male patients aged 20 years and older with a first diagnosis of thalassemia (ICD-9-CM code 282.4) between 1998 and 2010 from the LHID 2000 to be the thalassemia cohort. The index date for each thalassemia case was the date of diagnosis. To increase statistical power, for each case of thalassemia, 4 male control subjects without thalassemia were selected from the LHID 2000 as the nonthalassemia cohort, and were frequency-matched for age (in 5-year bands) and the year of diagnosis. Patients from both cohorts with organic ED (ICD-9-CM code 607.84) or sickle cell, iron deficiency anemia (ICD-9-CM codes 280), other deficiency anemias (ICD-9-CM codes 281), aplastic, hemolytic, or sideroblastic anemia, anemia of chronic disease (ICD-9-CM codes 282.6–285.8), myelodysplastic syndrome (ICD-9-CM codes 238.7), primary or secondary hemochromatosis (ICD-9-CM codes 275.0), or hematological malignancies (ICD-9-CM codes 200–208), before the index date, were excluded. In addition, we excluded cases with a history of transfusion (ICD-9 procedure codes 990) or with missing age or sex information at the baseline. There were no patients in whom age information was lacking.
Outcome and Comorbidities
Follow-up was calculated in person-years for each patient until organic ED was diagnosed, death occurred, the patient withdrew from the insurance system, or until the end of 2011. Organic ED was diagnosed based on the results of a self-administered International Index of Erectile Dysfunction (IIEF-5) questionnaire. The questionnaire was given to patients who complained of ED symptoms or had a clinical impression of ED. In addition, patients had received a diagnosis of ED on 2 separate occasions, and at least one of the diagnoses was made by an Urologist during the period between 2001 and 2011.
We also analyzed baseline comorbidities thought to be associated with organic ED: anemia (ICD-9-CM code 285.9), anxiety (ICD-9-CM code 300.00), coronary artery disease (ICD-9-CM codes 410–414), cirrhosis (ICD-9-CM code 571), chronic kidney disease (CKD) (ICD-9-CM codes 580–589), chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes 490–496), diabetes (ICD-9-CM code 250), depression (ICD-9-CM codes 296.2, 296.3, 300.4, 311), hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM code 272), and peripheral artery disease (ICD-9-CM codes 443.81, 443.9, 440.2, 444.2, 444.89).
Statistical Analysis
The distributions of age and history of comorbidities were compared between the thalassemia and nonthalassemia cohorts. The differences between categorical variables were analyzed using the chi-square test, and differences in continuous variables were estimated using t tests. The incidence densities rate of organic ED was calculated by age and comorbidity for each cohort. Univariable and multivariable Cox proportional hazard regression models were used to assess the risk of organic and psychogenic ED associated with thalassemia, compared with the nonthalassemia cohort. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated in the Cox model. Multivariate models were adjusted for age and comorbidities of cirrhosis, CAD, CKD, COPD, diabetes, hypertension, hyperlipidemia, and anxiety. We used the Kaplan–Meier method to compare the cumulative incidence of organic and psychogenic ED events between the 2 cohorts, and used the log-rank test to examine the differences. All data analyses were performed using the SAS 9.3 statistical package (SAS Institute Inc, NC), with P < 0.05 in 2-tailed tests considered significant.
RESULTS
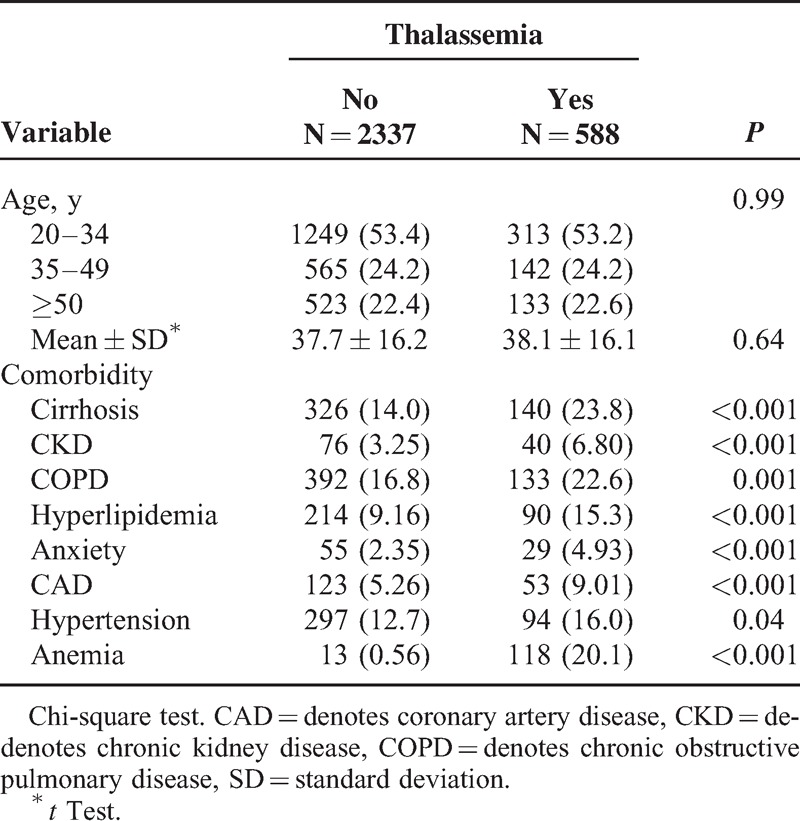
A total of 588 thalassemia male and 2337 male controls without thalassemia were included in our study. The mean ages in the thalassemia and nonthalassemia cohorts were 38.1 ± 16.1 years and 37.7 ± 16.2 years, respectively. The thalassemia cohort had more prevalence of comorbidities including cirrhosis (23.8% vs 14.0%), CKD (6.80% vs 3.25%), COPD (22.6% vs 16.8%), hyperlipidemia (15.3% vs 9.16%), anxiety (4.93% vs 2.35%), coronary artery disease (9.01% vs 5.26%), hypertension (16.0% vs 12.7%), and anemia (20.1% vs 0.56%) than did the nonthalassemia cohort (all P < .05). (Table 1)
TABLE 1.
Demographic Characteristics and Comorbidities in Cohorts With and Without Thalassemia
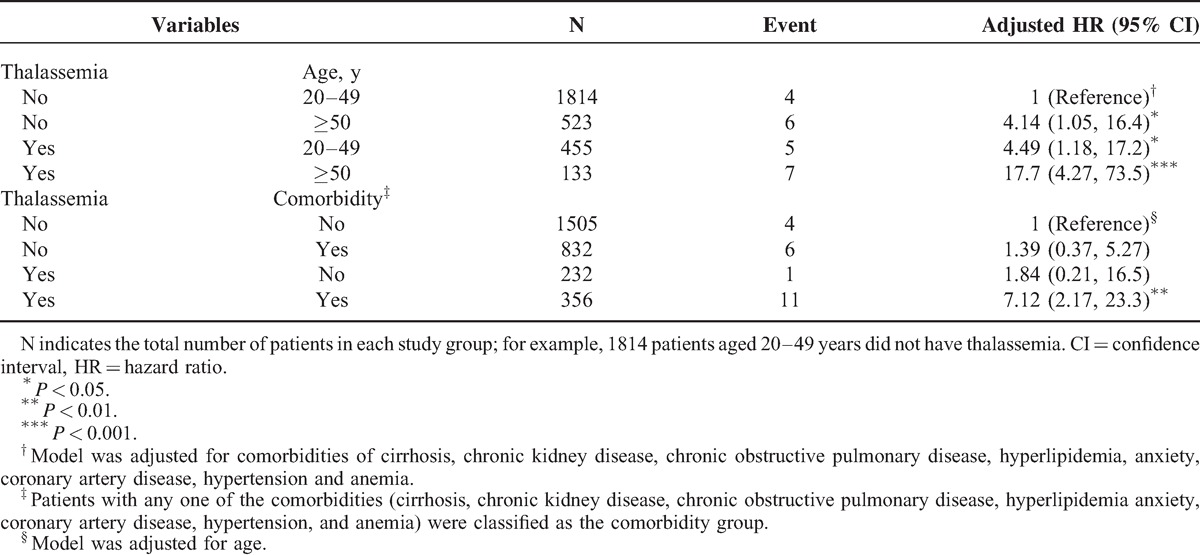
The mean follow-up years were 7.27 (SD = 3.51) and 7.22 (SD = 3.48) for the thalassemia cohort and the nonthalassemia cohort, respectively (data not shown). Figure 1 shows that the cumulative incidence of organic and psychogenic ED was higher in the thalassemia cohort than in the nonthalassemia cohort by 1.88% (log-rank test P < .001) at the end of follow-up. The overall incidence of organic ED was 4.73-fold higher in the thalassemia cohort than in the nonthalassemia cohort (2.81 and 0.59 per 1000 person-years, respectively), with an adjusted HR (aHR) of 4.56 (95% CI = 1.88–11.1) (Table 2). The organic ED incidence increased with age and with comorbidity in both cohorts. The age-specific thalassemia to nonthalassemia relative risk was the greater for all age group (aHR = 4.76; 95% CI = 1.24–18.2 for aged 20–49 years; aHR = 4.29; 95% CI = 1.31–14.1 for aged ≥50 years). Among patients with comorbidities, thalassemia men had a higher risk of organic ED than did the nonthalassemia cohort (aHR = 5.06; 95% CI = 1.86–13.7). Furthermore, relative to those patients without thalassemia and aged 20 to 49 years, patients with thalassemia and aged ≥50 years were 17.7-fold more likely to develop organic ED (95% CI = 4.27–73.5) (Table 3). Compared with patients without thalassemia and without comorbidities, cases with thalassemia and with comorbidities were 7.12-fold more likely to develop organic ED (95% CI = 2.17–23.3).
FIGURE 1.
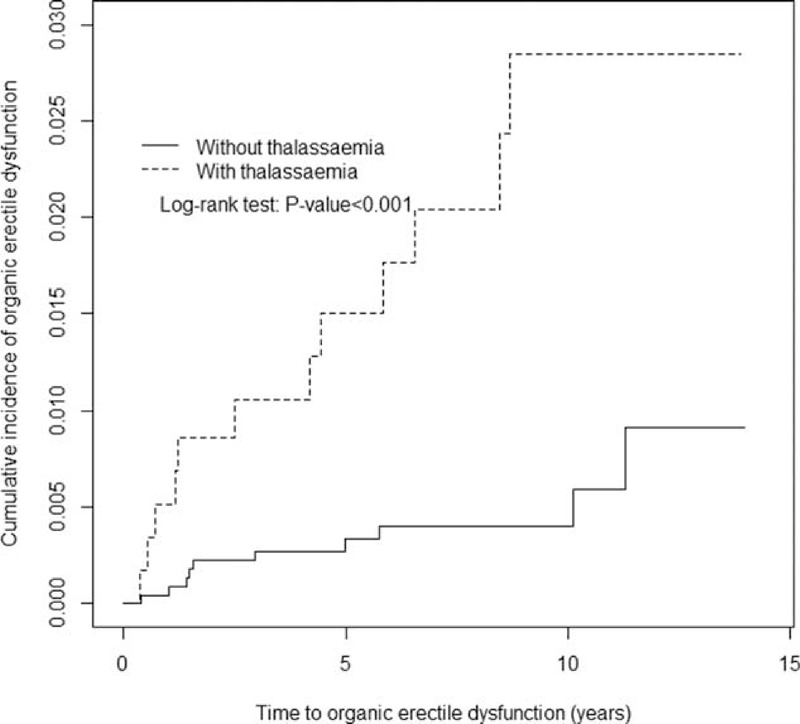
Cummulative incidence comparison of organic erectile dysfunction for patients with (dashed line) or without (solid line) thalassemia disease.
TABLE 2.
Incidence of Organic Erectile Dysfunction by Age and Comorbidity and Cox Model Measured Hazards Ratio for Patients With Thalassemia Compared With Those Without Thalassemia
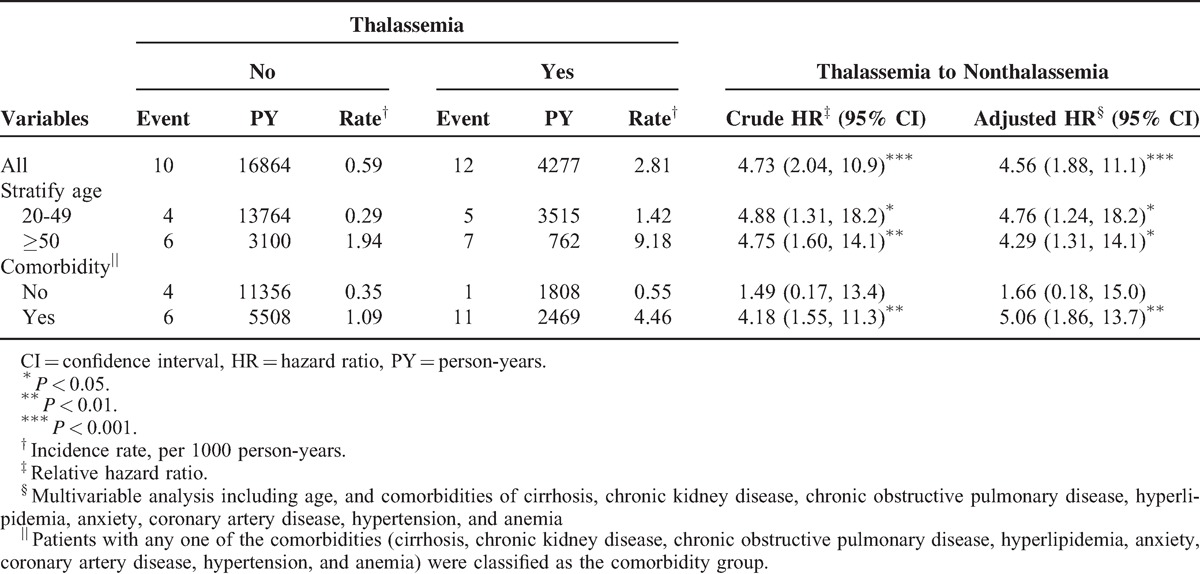
TABLE 3.
Cox Proportional Hazard Regression Analysis for the Risk of Organic Erectile Dysfunction-associated Thalassemia With Joint Effect of Age and Comorbidity
DISCUSSION
This is the first nationwide population-based cohort study based on an extremely large database and adjusted for numerous classic organic ED risk factors. This study suggests that transfusion-naive thalassemia men have an increased risk of developing ED. This risk was increased by 4.56-fold compared with the nonthalassemia controls after adjusting for age and medical comorbidities. According to the previous epidemiological results of thalassemia in Taiwan (our cohort database), the prevalence rates of α- and β-thalassemia were approximately 3% to 5% and 1% to 3%, respectively. Most patients with α- and β-thalassemia had hereditary traits or were carriers of borderline asymptomatic anemia or minimal symptoms, and were not transfusion-dependent.11,12 In addition, to purify our population using ICD procedure codes, we initially excluded male populations with transfusion-dependent thalassemia. This means that we excluded patients with thalassaemia major, who were likely to have hypogonadism as a result of abnormal iron deposition.13
Overall, according to our study results, the incidence rate of ED was higher among transfusion-naive thalassemia older men than among younger healthy men. In the patients with transfusion-naive thalassemia and chronic medical comorbidities, we also found that the incidence rate of organic ED was higher than among normal populations without chronic comorbidities. These results indicate that age and medical comorbidities play crucial roles in the development of ED in patients with transfusion-naive thalassemia. However, it is not clear from this study whether the thalassaemia magnifies this risk.
Most patients with thalassemia in our country were silent thalassemia carriers or had concealed thalassemia traits.14,15 Although relatively more cases were included in the present study than in other previous studies, it is likely that the patients in our study who were diagnosed with thalassemia had more severe clinical features and presented to medical services with complications, which led to the diagnosis of their thalassemia; or that these patients presented with nonhematological diagnoses and then were subsequently found to also have thalassemia during blood tests performed to investigate the presenting condition. Although more thalassaemia patients were included in this study, still very small numbers of these had ED. Only 12 patients had both diagnoses of thalassemia and ED, and only 10 patients in the nonthalassemic group had ED. Our results suggest that the small number of cases could lead to overemphasis of the results in this study. Therefore, in view of the small numbers, caution should be used in interpreting the findings of this study. Further studies, ideally prospective, should be done to investigate whether there is a true relationship between thalassaemia trait/NTDT and ED.
The process of erection is complex and involves numerous vascular structures. Two major mechanisms that may affect the pathophysiological mechanism of ED are systemic organ and psychological effects. Penile vascular disease is the hallmark of the pathophysiological mechanism of organic ED.16 Vascular abnormalities include decreased arterial blood flow, ischemia-induced vascular hypoperfusion, subsequent increased cavernosal smooth-muscle contraction, or veno-occlusive dysfunction. These effects may result in eventual endothelial dysfunction.17 Therefore, traditional risk factors that impair the vascular system include several systemic diseases such as diabetes mellitus, hypertension, dyslipidemia, end-stage kidney disease, cirrhosis of the liver, and coronary artery disease.18–20 Several studies have also indicated that ED could be an early sign of endothelial dysfunction and microvascular disease.21–23
Several hypothetical effects may explain the association between organic ED and transfusion-naive thalassemia. In patients with thalassemia who have less severe clinical manifestations, anemic status is a possible contributory factor to ED. However, although the patients in our study had transfusion-naive thalassemia, few NTDT patients who did not require any blood transfusions to maintain activities of daily life existed in our study populations. NTDT patients exhibit ineffective erythropoiesis and hemolytic anemia, which results from an imbalance of the ratio between α and β associated with membrane damage. 24,25 Despite disease genetic polymorphisms related to various clinical manifestations and disease severity, persistent membrane destruction related to hemolysis results in low arginine and nitric oxide (NO) bioavailability, as well as superoxide production with oxidative stress and endothelial inflammation.26 A recent study with β-thalassemic mice showed a decrease in NO bioavailability in vascular endothelial cells and impairment in preserved smooth muscle cell reactivity to NO. In addition, Butthep et al found vascular endothelial cell injury/dysfunction in patients with α- and β-thalassemia and a relative decrease in protein C and S levels but an increased plasma thrombomodulin circulation of endothelial cells, vascular endothelial growth factor concentration, and tumor necrosis factor-alpha in circulation.27,28 These effects could result in vascular endothelial dysfunction, and subsequently decrease wall stress and cause softening of the vessel wall.29 Moreover, hemolytic anemia-related persistent endovascular dysfunction played a more crucial role in the vasculopathy of organic ED. This may explain why elderly thalassmic patients experienced higher rates of organic ED than younger patients. In addition, NTDT patients could have abnormal iron metabolism and hyperferritinemia with iron overload-related complications, including hypogonadism and endocrine abnormalities.30
This study has several limitations. First, the NHIRD does not contain detailed information regarding patients’ current use of medications such as psychological agents or hormonesuppressive therapy, smoking habits, or obesity, all of which might increase ED, whether in thalassemia or nonthalassemia populations, and are confounding factors in this study. Second, the NHRI database uses ICD-9 CM codes that do not reflect specific genotypes and associated hemoglobinopathies, which might have led to heterogeneous clinical manifestations. Certain detailed information of the patients with thalassemia, including hemoglobin level, clinical manifestations, and chronic complications, was not available. Detailed individual differences should be elucidated through further studies. Third, because the diagnosis of ED resulted from data collected from a self-administered IIEF-5 questionnaire, the incidence rate of ED might have been underestimated. ED patients in Taiwan are less likely to visit sexual specialists because of cultural taboos. Fourth, the evidence derived from retrospective cohort studies is typically lower in statistical quality than that derived from randomized trials because of potential biases related to adjustments for confounding variables. Fifth, more chronic comorbidities were observed in the thalassemic group, and the cause of organic ED might have been these comorbidities rather than thalassemia itself. The detailed causes were not clear, and further studies are necessary to elucidate the clinical correlation. This study does not provide strong evidence to differentiate between the relationship of thalassemia and ED, or comorbidities and ED. Sixth, in the analysis of comorbidities, the psychological causes of ED (ie, anxiety and depression) were particularly likely to be underdiagnosed due to under-reporting. Seventh, although the control groups selected were from those populations without the diagnosis of thalassemia, it does not mean that there were no thalassaemia cases in the control group. It could mean either that they have been tested and found not to have it, or that they have not been tested for it. Finally, only 20.1% of patients with thalassemia cohort have anemia. It did not contain the detailed information regarding those remainder patients were either with hematological featuring microcytsis only or anemia not being recorded in the diagnostic list.
The main contribution of our study is its use of a nationwide population-based database that contains data on a high number of thalassemia cases, and the results indicate that patients with transfusion-naive thalassemia exhibited a 4.56-fold risk of developing organic ED when compared with the general population. Higher incidence rates of organic ED were also observed in elderly patients with transfusion-naive thalassemia and chronic comorbidities. Although the detailed pathophysiological mechanism between organic ED and thalassemia may require further examination, we recommend that physicians consider the possibility of organic ED. In addition, more detailed longitudinal cohort studies are necessary to elucidate whether healthy carriers of thalassemia, including those with thalassemia minor/traits, may still be at risk of developing ED.
UNCITED REFERENCE
Acknowledgments
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002, Taiwan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Footnotes
Abbreviations: CIs = confidence intervals, ED = erectile dysfunction, HRs = hazard ratios, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, IIEF-5 = International Index of Erectile Dysfunction, IRR = incidence rate ratio, LHID 2000 = The Longitudinal Health Insurance Database 2000, NHIRD = National Health Insurance Research Database, NHRI = the National Health Research Institutes, NTDT = nontransfusion-dependent thalassemia.
Author contributions: conception and design, Y-GC and C-HK; administrative support, C-HK; collection and assembly of data, all authors; data analysis and interpretation, Y-GC, C-LL, and C-HK; manuscript writing, all authors; final approval of manuscript, all authors.
All authors have no conflicts of interest to declare.
REFERENCES
- 1.Lewis RW, Fugl-Meyer KS, Corona G, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med 2010; 7:1598–1607. [DOI] [PubMed] [Google Scholar]
- 2.Bacon CG, Mittleman MA, Kawachi I, et al. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med 2003; 139:161–168. [DOI] [PubMed] [Google Scholar]
- 3.Holden CA, McLachlan RI, Pitts M, et al. Men in Australia Telephone Survey (MATeS): a national survey of the reproductive health and concerns of middle-aged and older Australian men. Lancet 2005; 366:218–224. [DOI] [PubMed] [Google Scholar]
- 4.Bodie J, Lewis J, Schow D, et al. Laboratory evaluations of erectile dysfunction: an evidence based approach. J Urol 2003; 169:2262–2264. [DOI] [PubMed] [Google Scholar]
- 5.Chew KK. Prevalence of erectile dysfunction in community-based studies. Int J Impot Res 2004; 16:201–202. [DOI] [PubMed] [Google Scholar]
- 6.Weber MF, Smith DP, O’Connell DL, et al. Risk factors for erectile dysfunction in a cohort of 108 477 Australian men. Med J Aust 2013; 199:107–111. [DOI] [PubMed] [Google Scholar]
- 7.Weatherall DJ. The definition and epidemiology of non-transfusion-dependent thalassemia. Blood Rev 2012; 26 Suppl 1:S3–6. [DOI] [PubMed] [Google Scholar]
- 8.Musallam KM, Rivella S, Vichinsky E, et al. Non-transfusion-dependent thalassemias. Haematologica 2013; 98:833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borgna-Pignatti C, Gamberini MR. Complications of thalassemia major and their treatment. Exp Rev Hematol 2011; 4:353–366. [DOI] [PubMed] [Google Scholar]
- 10.Chen YC, Wu JC, Haschler I, et al. Academic impact of a public electronic health database: bibliometric analysis of studies using the general practice research database. PloS One 2011; 6:e21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko TM, Xu X. Molecular study and prenatal diagnosis of alpha- and beta-thalassemias in Chinese. J Formos Med Assoc 1998; 97:5–15. [PubMed] [Google Scholar]
- 12.Peng CT, Liu SC, Peng YC, et al. Distribution of thalassemias and associated hemoglobinopathies identified by prenatal diagnosis in Taiwan. Blood Cell Mol Dis 2013; 51:138–141. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham MJ, Macklin EA, Neufeld EJ, et al. Thalassemia Clinical Research N. Complications of beta-thalassemia major in North America. Blood 2004; 104:34–39. [DOI] [PubMed] [Google Scholar]
- 14.Lin HJ, Shih MC, Peng CT, et al. Hematological features and molecular lesions of hemoglobin gene disorders in Taiwanese patients. Int J Lab Hematol 2010; 32:1–7. [DOI] [PubMed] [Google Scholar]
- 15.Liu SC, Peng CT, Lin TH, et al. Molecular lesion frequency of hemoglobin gene disorders in Taiwan. Hemoglobin 2011; 35:228–236. [DOI] [PubMed] [Google Scholar]
- 16.McMahon CG. Erectile dysfunction. Int Med J 2014; 44:18–26. [DOI] [PubMed] [Google Scholar]
- 17.Saenz de Tejada I, Goldstein I, Azadzoi K, et al. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med 1989; 320:1025–1030. [DOI] [PubMed] [Google Scholar]
- 18.Shen YC, Weng SF, Wang JJ, et al. Erectile dysfunction and risk of end stage renal disease requiring dialysis: a nationwide population-based study. PloS One 2014; 9:e102055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adekanle O, Ndububa DA, Orji EO, et al. Assessment of the sexual functions of males with chronic liver disease in South West Nigeria. Ann Afr Med 2014; 13:81–86. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg AE, Eisenberg M, Patel CJ, et al. Diabetes severity, metabolic syndrome, and the risk of erectile dysfunction. J Sex Med 2013; 10:3102–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brock G. Diagnosing erectile dysfunction could save your patient's life. Can Urol Assoc J 2014; 8:S151–S152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reriani M, Flammer AJ, Li J, et al. Microvascular endothelial dysfunction predicts the development of erectile dysfunction in men with coronary atherosclerosis without critical stenoses. Coron Artery Dis 2014; 25:552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlachopoulos C, Ioakeimidis N, Aznaouridis K, et al. Prediction of cardiovascular events with aortic stiffness in patients with erectile dysfunction. Hypertension 2014; 64:672–678. [DOI] [PubMed] [Google Scholar]
- 24.Taher AT, Porter JB, Viprakasit V, et al. Defining serum ferritin thresholds to predict clinically relevant liver iron concentrations for guiding deferasirox therapy when MRI is unavailable in patients with non-transfusion-dependent thalassaemia. Br J Haematol 2015; 168:284–290. [DOI] [PubMed] [Google Scholar]
- 25.Higgs DR, Engel JD, Stamatoyannopoulos G. Thalassaemia. Lancet 2012; 379:373–383. [DOI] [PubMed] [Google Scholar]
- 26.Morris CR. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology /the Education Program of the American Society of Hematology American Society of Hematology Education Program 2008:177-85. [DOI] [PubMed] [Google Scholar]
- 27.Butthep P, Bunyaratvej A, Funahara Y, et al. Alterations in vascular endothelial cell-related plasma proteins in thalassaemic patients and their correlation with clinical symptoms. Thromb Haemost 1995; 74:1045–1049. [PubMed] [Google Scholar]
- 28.Butthep P, Rummavas S, Wisedpanichkij R, et al. Increased circulating activated endothelial cells, vascular endothelial growth factor, and tumor necrosis factor in thalassemia. Am J Hematol 2002; 70:100–106. [DOI] [PubMed] [Google Scholar]
- 29.Stoyanova E, Trudel M, Felfly H, et al. Vascular endothelial dysfunction in beta-thalassemia occurs despite increased eNOS expression and preserved vascular smooth muscle cell reactivity to NO. PloS One 2012; 7:e38089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musallam KM, Cappellini MD, Wood JC, et al. Iron overload in non-transfusion-dependent thalassemia: a clinical perspective. Blood Rev 2012; 26 Suppl 1:S16–S19. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig W, Phillips M. Organic causes of erectile dysfunction in men under 40. Urol Int 2014; 92:1–6. [DOI] [PubMed] [Google Scholar]
- 32.Kovac JR, Labbate C, Ramasamy R, et al. Effects of cigarette smoking on erectile dysfunction. Andrologia 2014; doi: 10.1111/and.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papagiannopoulos D, Khare N, Nehra A. Evaluation of young men with organic erectile dysfunction. Asian J Androl 2015; 17:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maiorino MI, Bellastella G, Esposito K. Lifestyle modifications and erectile dysfunction: what can be expected? Asian J Androl 2015; 17:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


