Abstract
Many studies suggest that catalase C-262T gene polymorphism is associated with cancer risk, but with inconsistent results. This study aimed to summarize the overall association between catalase C-262T polymorphism and cancer risk. Literature search was performed in PubMed, Embase, and other databases, studies regarding the association between catalase C-262T polymorphism and cancer risk were identified, and data were retrieved and analyzed by using Review Manager 5.0.24 and STATA 12.0. A total of 18 publications with 22 case–control studies, including 9777 cancer patients and 12,223 controls, met the inclusion criteria. Meta-analysis results showed significant association between catalase C-262 T polymorphism and cancer risk (TT vs CT + CC: odds ratio [OR] = 1.17, 95% confidence interval [CI] = 1.03–1.31, P = 0.01). Subgroup analyses stratified by cancer types suggested the catalase C-262T polymorphism was significantly associated with an increased prostate cancer risk (TT vs CT + CC: OR = 1.61, 95% CI = 1.17–2.22, P = 0.004); for subgroup analyses stratified by ethnicity, no associations between this polymorphism and Asians or whites were identified (CT + TT vs CC: OR = 1.11, 95% CI = 0.98–1.26, P = 0.09 for whites; OR = 1.19, 95% CI = 0.78–1.80, P = 0.42 for Asians). In summary, the catalase C-262T polymorphism may be a risk factor for cancer with cancer type-specific effects. Further studies should be performed to confirm these findings.
INTRODUCTION
Caner is one of the leading causes of death and a severe public health problem worldwide.1 However, the exact mechanism of carcinogenesis has not been fully elucidated yet, growing studies reported that the reactive oxygen species (ROS) contributes to various aspects of malignant tumors, including carcinogenesis, aberrant growth, metastasis, and angiogenesis.2 ROS-mediated damage to cellular macromolecules is believed to accumulate as a function of age and to lead to deleterious effects associated with carcinogenesis.3–4 The catalase (CAT) is an important enzyme involved in the production and dismutation of ROS,5 which can neutralize reactive oxygen species by converting H2O2 into H2O and O2. Some investigators reported a significant reduction of CAT activity in prostate cancer and lung cancer, implicating the possible role of CAT in the carcinogenesis.6–8
In humans, the CAT gene is encoded by the nuclear chromosome 11p13. The rs1001179 polymorphism (C-262T) of this gene is located on the promoter region and influences transcription factors-binding, altering the basal transcription and consequent expression of this enzyme.9 Compared with the C allele, the variant T allele of the CAT C-262T gene polymorphism has been associated with lower enzyme activity and hence increased levels of ROS.10 Thus, it is plausible that the endogenous variability associated with this polymorphism may play a role in the host response to oxidative stress, which accordingly influences the development and progression of cancer. Up till now, a number of case–control studies have been performed to identify the association of CAT C-262T polymorphism with cancer risk; however, the results remain inconsistent and inconclusive.11–12 Since meta-analysis is a powerful tool for analyzing cumulative data from studies in which individual sample sizes are small and the statistical power is low,13 a meta-analysis based on current available independent studies was performed, which may provide the evidence for the overall association of CAT C-262T polymorphism with cancer susceptibility.
MATERIAL AND METHODS
Identification and Eligibility of Relevant Studies
The electronic databases PubMed, Embase, Web of Science, and Cochrane Library were searched using the Mesh terms: “catalase or CAT,” “polymorphism or variant or mutation,” and “cancer or tumor or carcinoma or malignancy” (Last search update October 15, 2014). Additional eligible studies on this topic were identified by a hand search of references of retrieved articles. If studies used partly overlapped subjects, the study with the largest sample size was selected. The languages were limited to English. Only the studies with complete data on comparison of frequency of the CAT C-262T polymorphism between controls and patients with cancer were selected, and the distribution of genotypes in the control group should be consistent with Hardy-Weinberg equilibrium (HWE). Animal studies, case reports, review articles, abstracts, editorials, reports with incomplete data, and studies based on pedigree data were excluded. Institutional review board approval was not required for this retrospective meta-analysis.
Data Extraction
Two investigators extracted all data independently according to the inclusion and exclusion criteria, and reached a consensus on all items. In case of disagreement, a third author assessed these articles and made the final decision. For one publication with several cancer types, each one was treated as a single study. From each study, the following information was extracted: first author's name, year of publication, country where the study was conducted, ethnicity of the study population, genotyping methods, total number of cancer cases and controls, and genotype distributions of cases and controls.
Statistical Analysis
Review Manager Software 5.0.24 (Cochrane Collaboration, Oxford, UK) and STATA 12.0 (Stata Corp., College Station, TX) software were used to perform all statistical analyses. The following genotype contrasts were evaluated: allelic contrast (T vs C), additive genetic model (TT vs CC), dominant genetic model (CT + TT vs CC), and recessive genetic model (TT vs CT + CC). In addition, we conducted subgroup analyses by cancer types and ethnicity. The association between CAT C-262T polymorphism and cancer risk was measured by the odds ratio (OR) with 95% confidence interval (95% CI). The significance of the pooled OR was determined by the Z test and P < 0.05 was considered as statistically significant. The heterogeneity across studies was calculated using the chi-squared-based Q-test and the inconsistency index I2 with 95% CI. When a significant Q-test (P < 0.1 or I2 > 50%) indicated heterogeneity among studies, the random-effects model was used to calculate the pooled OR; otherwise, the fixed-effects model was used.
Funnel plot asymmetry and Harbord test were used to determine the potential publication bias.14 Sensitivity analysis was performed by sequentially excluding individual studies and recalculating the results.15 HWE was tested by Pearson χ2 test with significance set at P < 0.05.
RESULTS
Characteristics of Eligible Studies
A total of 18 publications with 22 case–control studies, including 9777 cancer patients and 12,223 controls, met our inclusion criteria and were included in this meta-analysis.16–33 The study selection process was shown in Figure 1.
FIGURE 1.
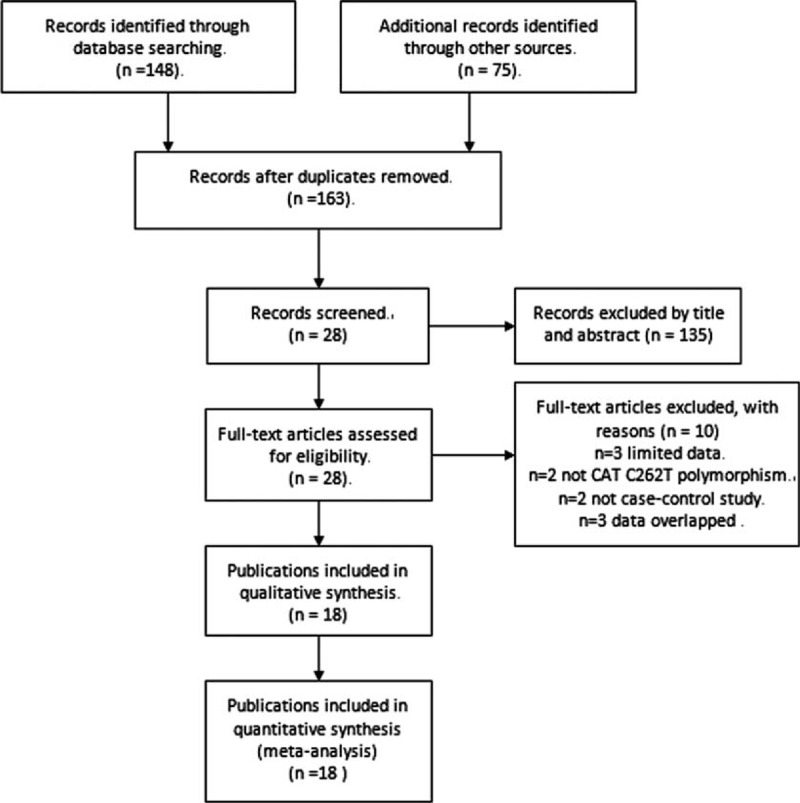
Flow of study identification, inclusion, and exclusion.
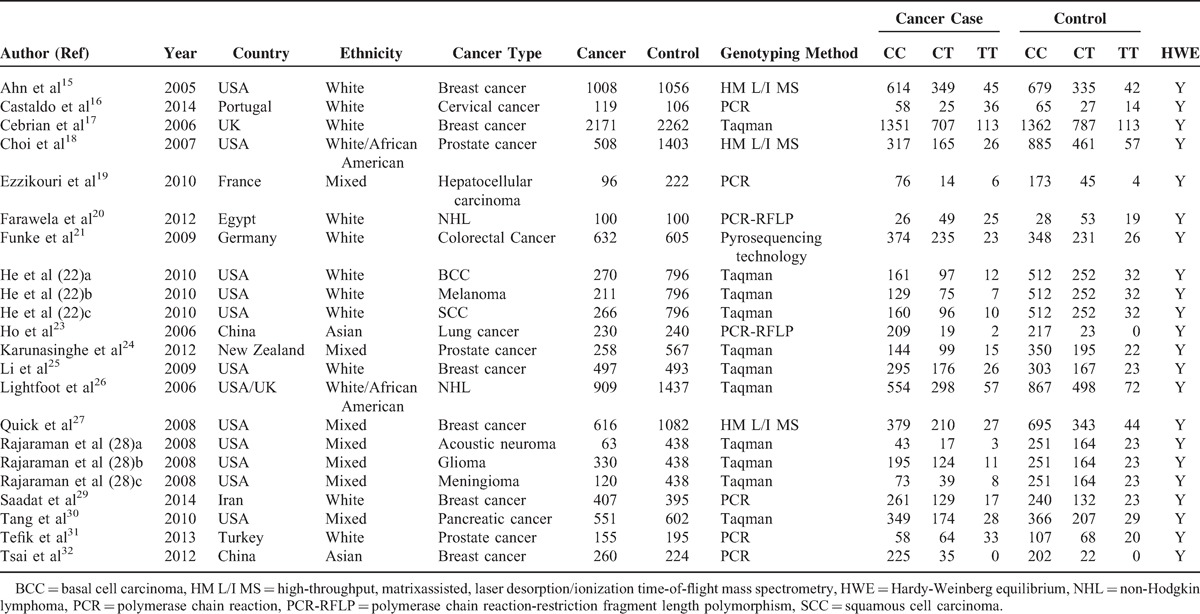
The included studies’ clinical characteristics and genotype distributions were summarized in Table 1. 15–32 These studies were published from 2005 to 2014. In all 22 studies, there were 11 studies of whites,16–18,21–23,26,30,32 2 studies of Asians,24,33 2 studies of whites and African-Americans,19,27 and 7 of mixed ethnicity.20,25,28,29,31 The 22 studies included 6 studies on breast cancer,16,18,26,28,30,33 3 studies on prostate cancer,19,25,32 3 studies on brain tumors (including acoustic neuroma, glioma, and meningioma),29 3 studies on skin cancer (including basal cell carcinoma, squamous cell carcinoma, and melanoma),23 2 studies on, non-Hodgkin lymphoma (NHL),21,27 1 study on hepatocellular carcinoma,20 1 study on colorectal cancer,22 1 study on lung cancer,24 1 study on cervical cancer,17 and 1 study on pancreatic cancer.31 The distributions of the genotypes in the control groups in all studies were in HWE. Genotyping methods used in the eligible studies included polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP),21,24 general PCR,17,20,30,32,33 Taqman,18,23,25–27,29,31 high-throughput, matrix-assisted, laser desorption/ionization time-of-flight mass spectrometry (HM L/I MS),16,19,28 and pyrosequencing technology,23 as listed in Table 1.
TABLE 1.
Clinical Summary of Included Studies and Genotype Distribution
Pooled Analysis
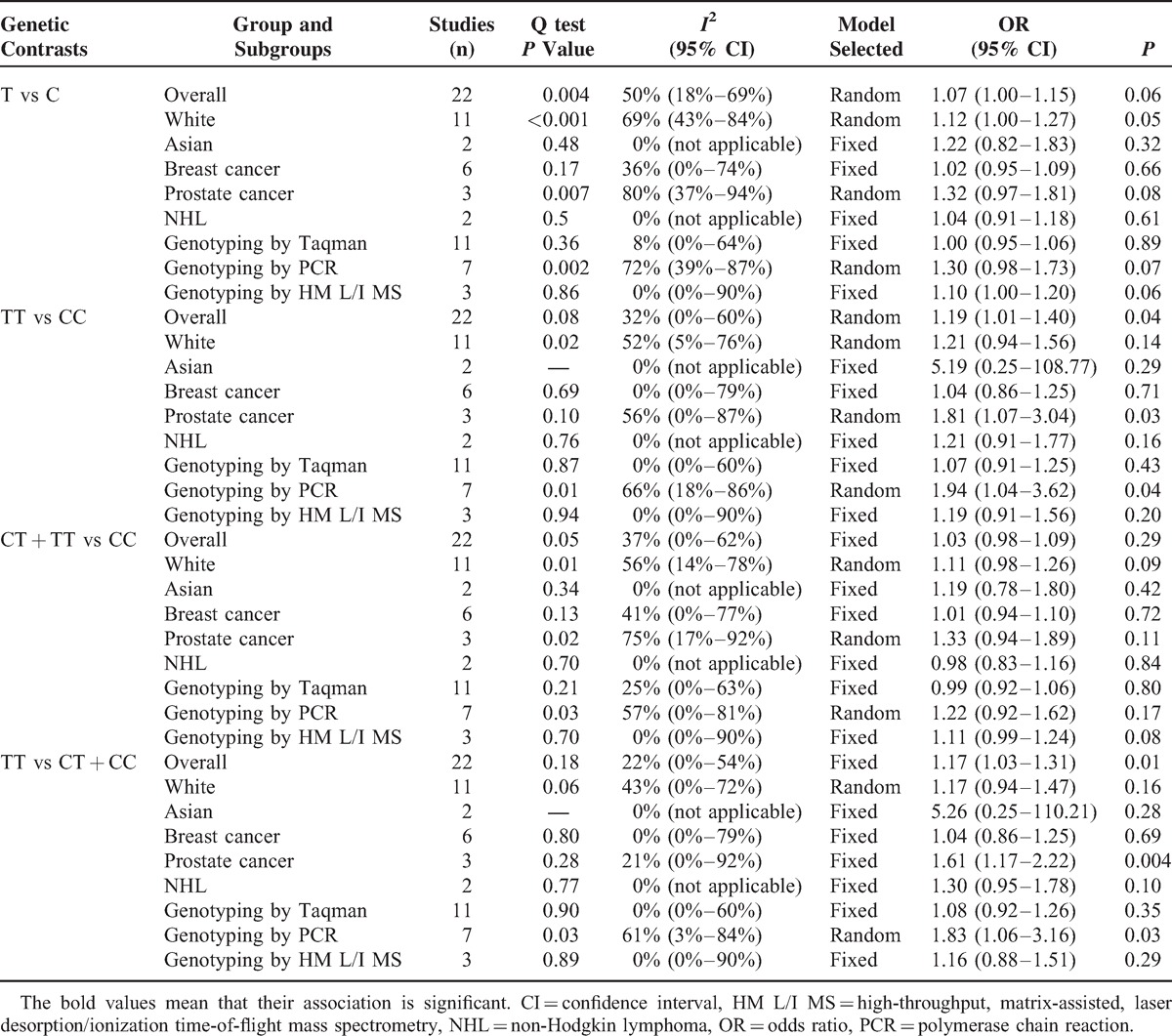
Meta-analysis results showed significant association between CAT C-262T polymorphism and the risk of cancer in additive and recessive genetic models (TT vs CC: OR = 1.19, 95% CI = 1.01–1.40, P = 0.04; TT vs CT + CC: OR = 1.17, 95% CI = 1.03–1.31, P = 0.01, Figure 2), but no evidence of association in other genetic models (T vs C: OR = 1.07, 95% CI = 1.00–1.15, P = 0.06; CT + TT vs CC: OR = 1.05, 95% CI = 0.97–1.13, P = 0.20). These results suggest that individuals who carry the TT homozygote may have an increased risk of cancer compared with the C allele carriers (CC or CT + CC).
FIGURE 2.
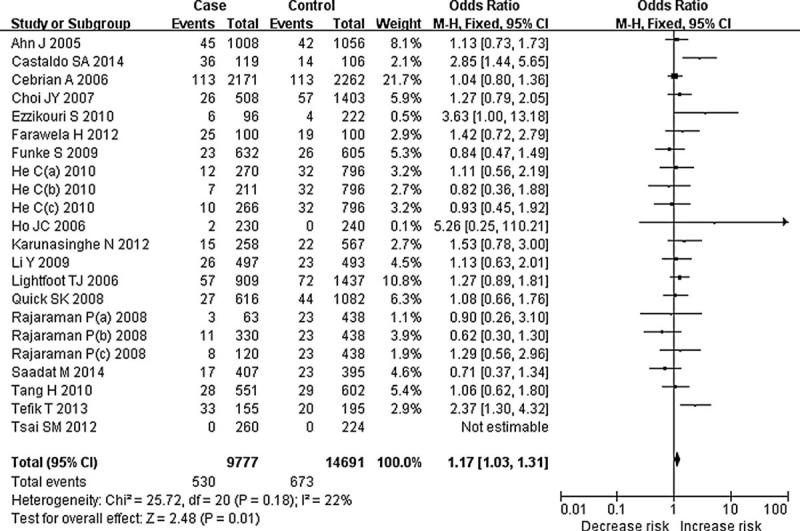
Forest plot for meta-analysis of catalase C-262T polymorphism and cancer risk (TT vs CT + CC). The size of the square is proportional to the weight of each study; horizontal lines represent the 95% confidence interval.
Subgroup Analysis
We then performed the subgroup analyses stratified by cancer types and ethnicity. The pooled ORs for additive model and recessive model comparison suggested the C-262T polymorphism was significantly associated with an increased prostate cancer risk (TT vs CC: OR = 1.81, 95% CI = 1.07–3.04, P = 0.03; TT vs CT + CC: OR = 1.61, 95% CI = 1.17–2.22, P = 0.004, Figure 3), whereas for breast cancer, NHL, such association was not significant in any genetic model (all P > 0.05). For subgroup analyses stratified by ethnicity, no associations between this polymorphism and Asian or white populations were identified (CT + TT vs CC: OR = 1.11, 95% CI = 0.98–1.26, P = 0.09 for white; CT + TT vs CC: OR = 1.19, 95% CI = 0.78–1.80, P = 0.42 for Asian) (Figure 4). These results suggest that the effects of CAT C-262T polymorphism on cancer susceptibility are ethnic and cancer subtype specific. Meanwhile, as the genotyping method may influence the results, we also performed a subgroup analysis according to genotyping method used in studies. Significant associations were only found in additive and recessive genetic models in studies using PCR (TT vs CC: OR = 1.94, 95% CI = 1.04–3.62, P = 0.04; TT vs CT + CC: OR = 1.83, 95% CI = 1.06–3.16, P = 0.03), whereas for studies using Taqman or HM L/I MS, no such associations were observed. The main results of the meta-analysis were summarized in Table 2.
FIGURE 3.
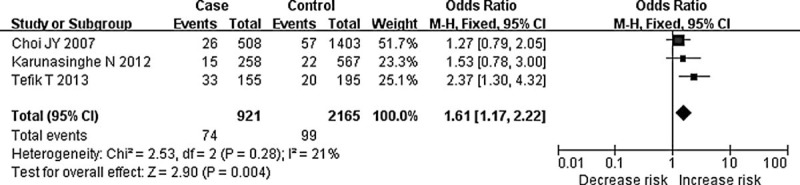
Forest plot for meta-analysis of catalase C-262T polymorphism and prostate cancer risk (TT vs CT + CC). The size of the square is proportional to the weight of each study; horizontal lines represent the 95% confidence interval.
FIGURE 4.
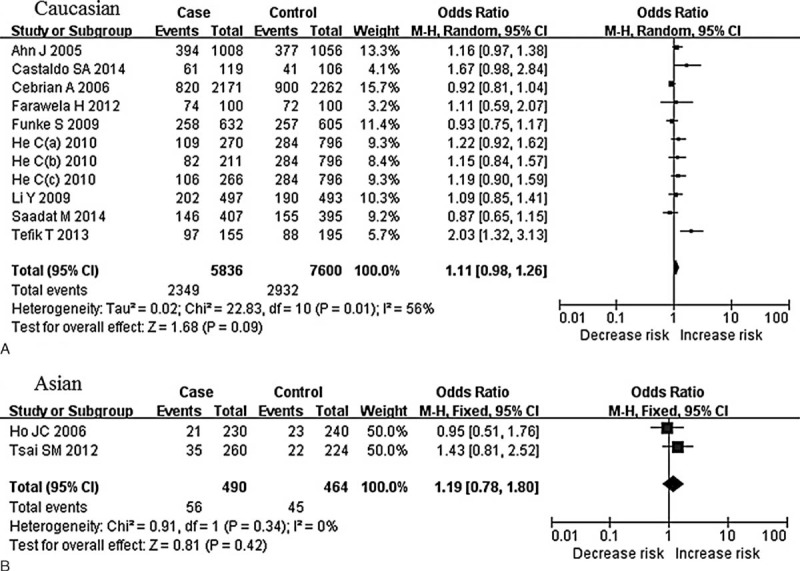
Forest plot for meta-analysis of catalase C-262T polymorphism and prostate cancer risk in white and Asian (CT + TT vs CC). The size of the square is proportional to the weight of each study; horizontal lines represent the 95% confidence interval. (A) White; (B) Asian.
TABLE 2.
Meta-analysis of Catalase C-262 T Polymorphism and Cancer Association
Publication Bias and Sensitivity Analysis
The publication bias of the studies was assessed by visual funnel plots and Harbord test. The funnel plots for CT + TT vs CC were shown in Figure 5 and Harbord test did not indicate asymmetry of the plot (P = 0.16), indicating a lack of publication bias. To evaluate the stability of our findings, sensitivity analysis was performed by sequentially excluding each study. Statistically similar results were obtained after sequentially excluding each study, suggesting the stability of the results (Figure 6).
FIGURE 5.
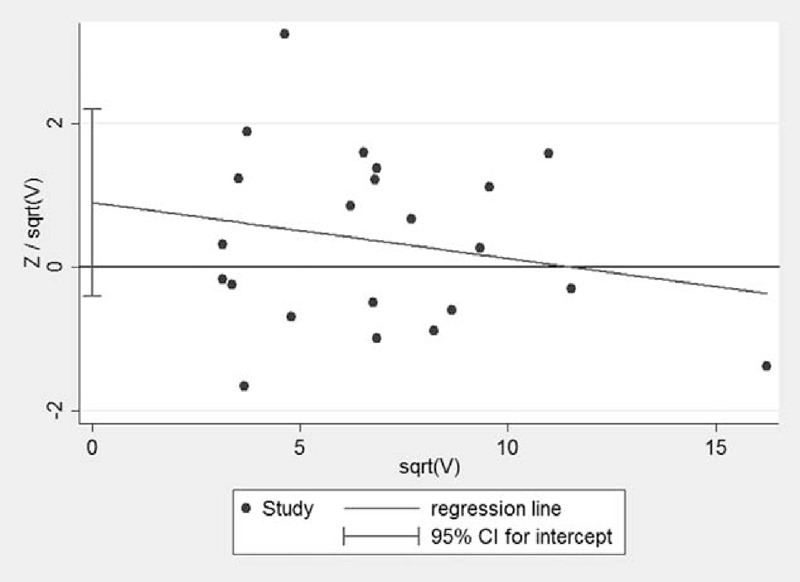
Funnel plot to detect publication bias.
FIGURE 6.
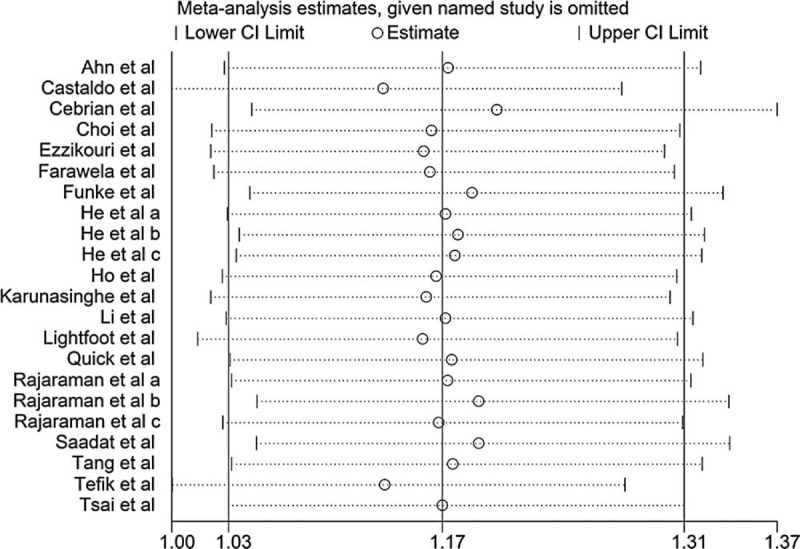
Sensitivity analysis of included studies.
DISCUSSION
CAT is a heme enzyme that plays a predominant role in controlling H2O2 concentration by converting H2O2 into H2O and O2, and protects cells from deleterious effects of oxidative stress34; studies suggest that CAT C-262T gene polymorphism influences transcription factors binding thus altering the basal transcription and consequent expression of this enzyme and hence the oxidative status of cells and its microenvironment.11,12 Therefore, this polymorphism is believed to play a role in the pathogenesis of cancer.11,12 As a number of studies have been published to investigate the potential association between CAT C-262T polymorphism and cancer risk with considerably variable results, we performed this meta-analysis to summarize their overall association.
The present meta-analysis included 18 publications with 22 case–control studies, comparisons of dominant/recessive/additive models and allele frequency were all estimated. In addition, the consistency of genetic effects across different ethnicities and cancer types was investigated. Based on current available evidences, the individuals who carry the TT homozygote have 17% increased risk of cancer compared with the C allele carriers, indicating that the CAT C-262T gene polymorphism may be a risk factor for cancer. Sensitivity analysis was performed to evaluate whether a single study influenced the overall results, and showed the stability and reliability of our statistical results.15
Although growing studies have suggested population-specific genetic differences in cancer pathogenesis, no association between CAT C-262T polymorphism and cancer risk was observed in our subgroup analysis stratified by ethnicity, which could be explained by that for certain population, cancer susceptibility may be associated with different genes, different loci within the same gene, and/or different polymorphisms at the same locus.35,36 In addition, 7 studies in our meta-analysis included population with mixed ethnicities,20,25,28,29,31 and we did not find studies performed in Latinos, so it is hard to make a definite conclusion about the population-specific genetic differences between the CAT polymorphism and cancer risk; further studies should pay attention to the ethnic-specific effects on cancer risk. Moreover, our results also showed significant association between C-262T gene polymorphism and increased prostate cancer risk, but not risks of other cancer types, revealing that although the etiology of cancers may overlap, the different cancers appear to have different genetic risk profiles and environmental factors may also contribute to at least part of the cancer subtype bias observed here in the association between the CAT C-262T polymorphism and cancer risk.37
It is worth mentioning a recent study by Tefik et al,32 which found that compared with the CC genotype, the TT genotype in CAT C-262T gene had a 1.94- and 3.83-fold increased risk for high-stage disease and metastasis, respectively, implying that this polymorphism may also be a risk factor in tumor progression and metastasis. In addition, numerous studies have paid attention to the potential of CAT in the treatment of cancer.38 It has been reported that inhibition of CAT with shRNA results in high H2O2 production with increased cell migration and invasion in CL1–0 cells,39 whereas CAT overexpression in mammary cancer cells leads to a less aggressive phenotype and an altered response to chemotherapy,40 suggesting that CAT-mediated oxidative stress might be an important therapeutic target in cancer, Therefore, to make a better understanding of CAT-related genetic, epigenetic, environmental, and clinical factors may also lead to more effective prevention and treatment of cancer.
There are several points that should be addressed in our meta-analysis. First, a relatively small number of studies and subjects were included in this meta-analysis, which may reduce the statistical power for identifying possible associations between the CAT C-262T polymorphism and cancer risk. Secondly, only published studies were included in this meta-analysis; unpublished data and ongoing studies were not sought. As studies reporting positive findings are more likely to be accepted for publication, this may lead to outcome reporting or publication bias, which brings inflation of the associations. Thirdly, lack of the original data of the reviewed studies limited our further investigation of potential interactions between genes because one gene may enhance or hinder the expression of another gene. Fourthly, in this study, we observed that gene-typing method may also influence the assay results; further studies should pay attention to these aspects. Last but not least, the included publications were majorly limited to Asian and white populations, so future work should examine other populations, such as Latinos.
CONCLUSION
In summary, our results suggest that the CAT C-262T gene polymorphism may be a risk factor for cancer with cancer type-specific effects. Large well-designed, multicenter epidemiological studies should be carried out in these and other ethnic populations to confirm our findings.
Acknowledgments
We are indebted to the authors of the primary studies included in this meta-analysis; without their contributions, this work would not have been possible.
Footnotes
Abbreviations: CAT = Catalase, HM L/I MS = High-throughput∗ matrixassisted∗ laser desorption/ionization time-of-flight mass spectrometry, HWE = Hardy-Weinberg equilibrium, OR = odds ratio, PCR-RFLP = polymerase chain reaction-restriction fragment length polymorphism, ROS = reactive oxygen species.
YS, DL, and PT contributed equally to this work and share joint first authorship.
Author contributions: YCS, DDL, and PWT conceived the article, systematic review, meta-analysis, drafted, and revised the manuscript; KLS, JZ, and MF contributed in literature search, systematic review, drafted, and revised the manuscript; CW, TY, and LC contributed in systematic review, drafted, and revised the manuscript; FQW is the guarantor of the article, taking responsibility for the integrity of the work as a whole, from inception to published article.
This work was supported by grants 81230001 and 81300032 from the National Natural Science Foundation of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no conflicts of interest.
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64:252–271. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer Lett 2008; 266:53–59. [DOI] [PubMed] [Google Scholar]
- 3.Sarsour EH, Kumar MG, Chaudhuri L, et al. Redox control of the cell cycle in health and disease. Antioxid Redox Signal 2009; 11:2985–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol 2014; 25:23–32. [DOI] [PubMed] [Google Scholar]
- 5.Laurent A, Nicco C, Chéreau C, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res 2005; 65:48–56. [PubMed] [Google Scholar]
- 6.Battisti V, Maders LD, Bagatini MD, et al. Oxidative stress and antioxidant status in prostate cancer patients: relation to Gleason score, treatment and bone metastasis. Biomed Pharmacother 2011; 65:516–524. [DOI] [PubMed] [Google Scholar]
- 7.Bostwick DG, Alexander EE, Singh R, et al. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer 2000; 89:123–134. [PubMed] [Google Scholar]
- 8.Zanini D, Pelinson LP, Schmatz R, et al. δ-aminolevulinate dehydratase activity in lung cancer patients and its relationship with oxidative stress. Biomed Pharmacother 2014; 68:603–609. [DOI] [PubMed] [Google Scholar]
- 9.Khodayari S, Salehi Z, Fakhrieh Asl S, et al. Catalase gene C-262T polymorphism: importance in ulcerative colitis. J Gastroenterol Hepatol 2013; 28:819–822. [DOI] [PubMed] [Google Scholar]
- 10.Ahn J, Nowell S, McCann SE, et al. Associations between catalase phenotype and genotype: modification by epidemiologic factors. Cancer Epidemiol Biomarkers Prev 2006; 15:1217–1222. [DOI] [PubMed] [Google Scholar]
- 11.Ding G, Liu F, Shen B, et al. The association between polymorphisms in prooxidant or antioxidant enzymes (myeloperoxidase, SOD2, and CAT) and genes and prostate cancer risk in the Chinese population of Han nationality. Clin Genitourin Cancer 2012; 10:251–255. [DOI] [PubMed] [Google Scholar]
- 12.Cheng TY, Barnett MJ, Kristal AR, et al. Genetic variation in myeloperoxidase modifies the association of serum δ-tocopherol with aggressive prostate cancer among current smokers. J Nutr 2011; 141:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panagiotou OA, Willer CJ, Hirschhorn JN, et al. The power of meta-analysis in genome-wide association studies. Annu Rev Genomics Hum Genet 2013; 14:441–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006; 25:3443–3457. [DOI] [PubMed] [Google Scholar]
- 15.Jia J, Ren J, Yan D, et al. Association Between the XRCC6 Polymorphisms and Cancer Risks: A Systematic Review and Meta-analysis. Medicine (Baltimore) 2015; 94:e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn J, Gammon MD, Santella RM, et al. Associations between breast cancer risk and the catalase genotype, fruit and vegetable consumption, and supplement use. Am J Epidemiol 2005; 162:943–952. [DOI] [PubMed] [Google Scholar]
- 17.Castaldo SA, da Silva AP, Matos A, et al. The role of CYBA (p22phox) and catalase genetic polymorphisms and their possible epistatic interaction in cervical cancer. Tumour Biol 2015; 36:909–914. [DOI] [PubMed] [Google Scholar]
- 18.Cebrian A, Pharoah PD, Ahmed S, et al. Tagging single-nucleotide polymorphisms in antioxidant defense enzymes and susceptibility to breast cancer. Cancer Res 2006; 66:1225–1233. [DOI] [PubMed] [Google Scholar]
- 19.Choi JY, Neuhouser ML, Barnett M, et al. Polymorphisms in oxidative stress-related genes are not associated with prostate cancer risk in heavy smokers. Cancer Epidemiol Biomarkers Prev 2007; 16:1115–1120. [DOI] [PubMed] [Google Scholar]
- 20.Ezzikouri S, El Feydi AE, Afifi R, et al. Polymorphisms in antioxidant defence genes and susceptibility to hepatocellular carcinoma in a Moroccan population. Free Radic Res 2010; 44:208–216. [DOI] [PubMed] [Google Scholar]
- 21.Farawela H, Khorshied M, Shaheen I, et al. The association between hepatitis C virus infection, genetic polymorphisms of oxidative stress genes and B-cell non-Hodgkin's lymphoma risk in Egypt. Infect Genet Evol 2012; 12:1189–1194. [DOI] [PubMed] [Google Scholar]
- 22.Funke S, Hoffmeister M, Brenner H, et al. Effect modification by smoking on the association between genetic polymorphisms in oxidative stress genes and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 2009; 18:2336–2338. [DOI] [PubMed] [Google Scholar]
- 23.He C, Qureshi AA, Han J. Polymorphisms in genes involved in oxidative stress and their interactions with lifestyle factors on skin cancer risk. J Dermatol Sci 2010; 60:54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho JC, Mak JC, Ho SP, et al. Manganese superoxide dismutase, catalase genetic polymorphisms, activity levels, lung cancer risk in Chinese in Hong Kong. J Thorac Oncol 2006; 1:648–653. [PubMed] [Google Scholar]
- 25.Karunasinghe N, Han DY, Goudie M, et al. Prostate disease risk factors among a New Zealand cohort. J Nutrigenet Nutrigenomics 2012; 5:339–351. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Ambrosone CB, McCullough MJ, et al. Oxidative stress-related genotypes, fruit and vegetable consumption and breast cancer risk. Carcinogenesis 2009; 30:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lightfoot TJ, Skibola CF, Smith AG, et al. Polymorphisms in the oxidative stress genes, superoxide dismutase, glutathione peroxidase and catalase and risk of non-Hodgkin's lymphoma. Haematologica 2006; 91:1222–1227. [PubMed] [Google Scholar]
- 28.Quick SK, Shields PG, Nie J, et al. Effect modification by catalase genotype suggests a role for oxidative stress in the association of hormone replacement therapy with postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 2008; 17:1082–1087. [DOI] [PubMed] [Google Scholar]
- 29.Rajaraman P, Hutchinson A, Rothman N, et al. Oxidative response gene polymorphisms and risk of adult brain tumors. Neuro Oncol 2008; 10:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saadat M, Saadat S. Genetic polymorphism of CAT C-262 T and susceptibility to breast cancer, a case-control study and meta-analysis of the literatures. Pathol Oncol Res 2014; DOI: 10. 1007/s12253-014-9840-4. [DOI] [PubMed] [Google Scholar]
- 31.Tang H, Dong X, Day RS, et al. Antioxidant genes, diabetes and dietary antioxidants in association with risk of pancreatic cancer. Carcinogenesis 2010; 31:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tefik T, Kucukgergin C, Sanli O, et al. Manganese superoxide dismutase Ile58Thr, catalase C-262T and myeloperoxidase G-463A gene polymorphisms in patients with prostate cancer: relation to advanced and metastatic disease. BJU Int 2013; 112:E406–414. [DOI] [PubMed] [Google Scholar]
- 33.Tsai SM, Wu SH, Hou MF, et al. Oxidative stress-related enzyme gene polymorphisms and susceptibility to breast cancer in non-smoking, non-alcohol-consuming Taiwanese women: a case-control study. Ann Clin Biochem 2012; 49:152–158. [DOI] [PubMed] [Google Scholar]
- 34.Bauer G. Tumor cell-protective catalase as a novel target for rational therapeutic approaches based on specific intercellular ROS signaling. Anticancer Res 2012; 32:2599–2624. [PubMed] [Google Scholar]
- 35.Ju W, Kim JW, Park NH, et al. Matrix metalloproteinase-1 promoter polymorphism and epithelial ovarian cancer: does ethnicity matter? J Obstet Gynaecol Res 2007; 33:155–160. [DOI] [PubMed] [Google Scholar]
- 36.Chen B, Zhou Y, Yang P, et al. CDH1-160C>A gene polymorphism is an ethnicity-dependent risk factor for gastric cancer. Cytokine 2011; 55:266–273. [DOI] [PubMed] [Google Scholar]
- 37.Danaei G, Vander Hoorn S, Lopez AD, et al. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005; 366:1784–1793. [DOI] [PubMed] [Google Scholar]
- 38.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 2013; 12:931–947. [DOI] [PubMed] [Google Scholar]
- 39.Tsai JY, Lee MJ, Dah-Tsyr Chang M, et al. The effect of catalase on migration and invasion of lung cancer cells by regulating the activities of cathepsin S, L, and K. Exp Cell Res 2014; 323:28–40. [DOI] [PubMed] [Google Scholar]
- 40.Glorieux C, Dejeans N, Sid B, et al. Catalase overexpression in mammary cancer cells leads to a less aggressive phenotype and an altered response to chemotherapy. Biochem Pharmacol 2011; 82:1384–1390. [DOI] [PubMed] [Google Scholar]


