FIGURE 1.
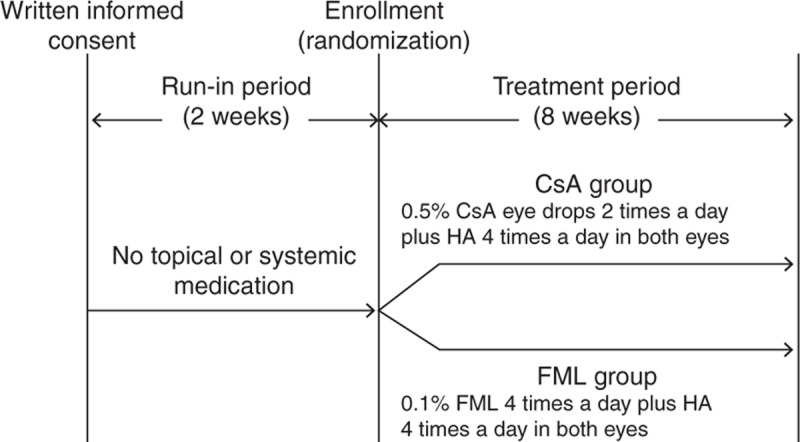
Study design. During the 2-week run-in period, the patients were instructed to abstain from the use of any topical ophthalmic medication. During the 8-week treatment period, both the groups were treated with an ophthalmic solution containing 0.1% HA 4 times per day. In addition, the CsA group was treated with an ophthalmic solution containing 0.5% CsA twice a day, and the FML group was treated with an ophthalmic solution containing 0.1% FML 4 times per day. Both the eyes of each patient were treated at all of the time points. CsA = cyclosporine, FML = fluorometholone, HA = sodium hyaluronate.
