Abstract
Background
Multiple studies have been conducted that demonstrate the superiority of patch angioplasty over primary closure for carotid endarterectomy (CEA). Patch angioplasty with poly-tetrafluorethylene patches (ACUSEAL) have shown results comparable to patch angioplasty with saphenous vein and polyester patches. This is a prospective randomized study to compare the clinical outcomes of CEA using ACUSEAL versus bovine pericardium patching (Vascu-Guard).
Methods
Two hundred patients were randomized (1:1) to either ACUSEAL or Vascu-Guard patching. Demographic data/clinical characteristics were collected. Intraoperative hemostasis times and the frequency of reexploration for neck hematoma were recorded. All patients received immediate and 1-month postoperative duplex ultrasound studies, which were repeated at 6-month intervals. A Kaplan–Meier analysis was used to estimate the risk of restenosis and the stroke-free survival rates.
Results
The demographics were similar in both groups, except for a higher incidence of current smokers in the ACUSEAL group and more patients with congestive heart failure in the Vascu-Guard group (P = 0.02 and 0.03, respectively). The mean operative internal carotid artery diameter and the mean arteriotomy length were similar in both groups. The mean hemostasis time was 4.90 min for ACUSEAL patching vs. 3.09 min for Vascu-Guard (P = 0.027); however, the mean operative times were similar for both groups (ACUSEAL 2.09 hr vs. Vascu-Guard 2.16 hr, P = 0.669). The incidence of reexploration for neck hematoma was higher in the Vascu-Guard group; 6.12% vs. 1.03% (P = 0.1183). The incidence of perioperative ipsilateral neurologic events was 3.09% for ACUSEAL patching vs. 1.02% for Vascu-Guard patching (P = 0.368). The mean follow-up period was 15 months. The respective freedom from ≥70% carotid restenosis at 1, 2, and 3 years were 100%, 100%, and 100% for ACUSEAL patching vs. 100%, 98%, and 98% for Vascu-Guard patching (P = 0.2478). The ipsilateral stroke-free rates at 1, 2, and 3 years were 96% for ACUSEAL and 99% for Vascu-Guard patching.
Conclusions
Although CEA patching with ACUSEAL versus Vascu-Guard differed in hemostasis time, the frequency of reexploration for neck hematomas was more frequent in the pericardial patch group; however, only 1 patient had documented suture line bleeding and the surgical reexploration rate is not likely to be patch related. There were not any significant differences in perioperative/late neurologic events and late restenosis in the 2 groups.
INTRODUCTION
In multiple prospective and retrospective clinical trials, including studies by our group, standard carotid endarterectomy (CEA) with patch closure has been shown to be safe, durable, and superior to primary closure. The use of patch angioplasty versus primary closure has been shown to reduce the incidence of perioperative stroke, perioperative carotid thrombosis, and long-term carotid artery restenosis.1–12
Several different patches are currently available, but the ideal patch is yet to be defined and controversies still exist regarding which patch is superior. Others have reported that certain patch materials may have a higher restenosis rate than venous patch closure. In addition, some institutions have shown that eversion endarterectomy can provide results similar to that of patch closure, so both techniques are widely accepted over primary closure of the arteriotomy.12,13
Several advantages of prosthetic patching over autogenous vein patching have been reported, such as decreased operative times, decreased incidence of aneurysmal dilatation or patch rupture, increased availability, and elimination of complications associated with autogenous vein harvesting.2,10
In an effort to provide further clinical data on patching of the carotid artery with different prosthetic conduits, we conducted a prospective randomized trial comparing the 2 most commonly implanted patches at our institution. Herein, we report the results of the prospective randomized trial comparing ACUSEAL (W. L. Gore & Associates, Flagstaff, AZ) versus bovine pericardium patches (Vascu-Guard; Synovis Life Technologies, Inc.; Biovascular, Inc., Saint Paul, MN) used during CEA.
METHODS
Patient Population
This prospective randomized study was done between September 2009 and January 2012. Two hundred CEA patients were consented in 28 months and randomized into 100 CEAs with ACUSEAL and 100 CEAs with Vascu-Guard patch, similar to previous studies conducted at our institution.17–19
Patients were randomized in a 1:1 ratio between the 2 surgical procedures using sealed opaque envelopes, each containing a slip of paper with the procedure assignment. The randomization envelopes were generated in blocks of 10 and placed in a closed container. After anesthesia was started, but before the first skin incision, an envelope was pulled from the container by the study controller and opened; and the surgeon was immediately notified of the procedure assignment.
During the study period, 5 patients were excluded from the trial for the following reasons: study patch was too short for the atherosclerotic lesion and required interposition repair (3 cases: 1 ACUSEAL and 2 Vascu-Guard), patient’s disease was too extensive (1 case: ACUSEAL), and patient’s internal carotid artery (ICA) was totally occluded at time of surgery before the endarterectomy (1 case: ACUSEAL). Of the 5 patients, 1 was excluded prior to randomization; but of the remaining 4 patients, 3 were randomized to ACUSEAL and 1 was randomized to the Vascu-Guard group. All patients who were excluded had duplex examinations only and the extent of disease extended into the common carotid artery, requiring interposition repair as described previously. In addition, the single patient with an intraoperative occlusion had a carotid duplex only and was falsely interpreted as a stenosis instead of an occlusion, which was seen intraoperatively. Therefore, only 195 patients who were randomized met the study criteria for follow-up examination: 97 patients in the ACUSEAL group and 98 patients in the Vascu-Guard group. This study was approved by the Charleston Area Medical Center/West Virginia University, Charleston Division, and Charleston, WV.
All patients underwent carotid color duplex ultrasound scanning, with or without magnetic resonance angiography (MRA), and computed tomography angiography (CTA) before the surgery to determine the degree of preoperative stenosis. Baseline blood cholesterol and triglyceride levels, along with the HDL (high-density lipoprotein) and LDL (low-density lipoprotein), were obtained. Various preoperative risk factors were determined, including smoking status, hypertension, diabetes mellitus, coronary artery disease, and the preoperative use of antiplatelet therapy. The indications for CEA were categorized into asymptomatic versus symptomatic. Symptomatic patients were further divided into hemispheric transient ischemic attack (TIA) symptoms, amaurosis fugax, and hemispheric strokes. Patients were continued on aspirin therapy before and after surgery, clopidogrel was stopped for 7 days before surgery in all patients. Patients on Coumadin therapy for atrial fibrillation were either bridged with heparin in the hospital before and after surgery or held at the discretion of the operating physician.
Operative Technique
All procedures were performed under general anesthesia with systemic heparin and routine shunting as previously described.2 During surgery, the normal ICA distal to the lesion was measured in millimeters with calipers. ACUSEAL, cardiovascular patches, and Vascu-Guard patches were sutured with 6-0 polyprolene sutures (Prolene; Ethicon, Somerville, NJ). Thrombin-soaked oxidized cellulose and digital pressure were applied to stop any bleeding points before closure in patients with both patches. Hemostasis time was defined as the time elapsed between placing the thrombin and gel foam on the suture line until hemostasis was achieved. If no thrombin and gel foam were required, then hemostasis time was recorded as 0 min. The gel foam was placed and removed at 3-min intervals. If hemostasis was not achieved, then the gel foam was reapplied to the patch and subsequently removed at 1-min intervals, with no patients receiving protamine for reversal at the conclusion of the procedure. All patients were administered dextran 40 at 25 mL/hr until 7AM on the day after surgery. Completion postoperative duplex ultrasound scanning was performed on all patients on postoperative day 1 and all patients were resumed on a home antiplatelet regimen.
Surveillance Protocol
All patients had clinical/neurologic examinations postoperatively and underwent postoperative color duplex ultrasound scanning the morning after surgery, which was repeated at 30 days, 6 months, and every 6 months thereafter. Peak systolic velocities of >274 cm/sec on duplex examination with spectral broadening throughout systole and an increased diastolic frequency were consistent with hemodynamically significant stenosis (70% reduction).3 Patients with an occlusion <30 days after the procedure were not considered in the analysis for late restenosis.
STATISTICAL METHODS
Morbidity rates and other noncontinuous variables were compared with either the chi-squared test or Fisher’s exact test. Perioperative complications were defined as complications occurring within 30 days of the CEA.14 Strokes were defined as ipsilateral territory permanent deficits and/or the presence of a new ipsilateral infarct on computed tomography (CT) or magnetic resonance imaging (MRI). Neck hematomas requiring surgical reexploration were included for analysis and comparison, whereas neck hematomas that required extension of hospitalization or required readmission were not included but were mentioned in the results section to aid in the description of such occurrences. A Kaplan–Meier primary analysis was used to calculate the occurrence of late events (time to >70% restenosis or stroke). Possible risk factors were chosen based on a univariate analysis with a one- or two-tailed P of 0.05. Models were then refined by multiple runs (forward and backward), eliminating factors unlikely to be associated with the outcome (i.e., high P value in these models).
RESULTS
There were no statistically significant differences in the demographics, other than current smokers (P = 0.029) in the ACUSEAL group and more patients with congestive heart failure in the Vascu-Guard group (P = 0.039). The clinical data that were statistically different between the groups included a higher total cholesterol level in the ACUSEAL group (P = 0.029), a longer hemostasis time of 4.9 min with the ACUSEAL and 3.09 min with the Vascu-Guard patches (P = 0.027). The arteriotomy length, ICA diameter, and surgery times were not statistically different between the 2 groups (Table I).
Table I.
Univariate analysis comparing both groups
| Characteristics | ACUSEAL (N = 97) | Bovine (N = 98) | P value |
|---|---|---|---|
| Age | 68.134 ± 10.7458 (43–94) | 67.449 ± 9.86 (37–84) | 0.6432 |
| Gender (male) | 54 (55.67%) | 53 (54.18%) | 0.8236 |
| Race (White) | 94 (97.92%) | 95 (100%) | 0.4974 |
| Preoperative laboratories | |||
| Total cholesterol | 175.3 ± 42.85 (108–355) | 161.1 ± 39.6 (97–296) | 0.0287a |
| LDL | 87.87 ± 35.38 (7–212) | 85.50 ± 31.32 (20–163) | 0.7048 |
| HDL | 46.20 ± 13.07 (25–85) | 44.96 ± 11.46 (24–83) | 0.5784 |
| Triglycerides | 204.6 ± 166.4 (60–1275) | 185.4 ± 144.2 (47–982) | 0.4282 |
| crp | 2.4917 ± 4.21 (0.2–15.5) | 5.02 ± 12.67 (0–41) | 0.5589 |
| Comorbidities | |||
| Current smoker | 47 (48.45%) | 31 (32.98%) | 0.0296a |
| Past smoker | 36 (38.71%) | 49 (52.69%) | 0.0557 |
| Diabetes mellitus | 34 (35.05%) | 37 (37.76%) | 0.6948 |
| Congestive heart failure | 4 (4.12%) | 12 (12.24%) | 0.0388a |
| Peripheral artery disease | 34 (35.05%) | 28 (28.57%) | 0.3313 |
| Hypertension | 12 (12.37%) | 18 (18.37%) | 0.2459 |
| Hypercholesterolemia | 76 (78.35%) | 82 (83.67%) | 0.3432 |
| Medications | |||
| ASA | 84 (88.42%) | 84 (85.71%) | 0.5756 |
| Plavix | 34 (36.56%) | 37 (39.36%) | 0.693 |
| Coumadin | 6 (6.67%) | 8 (8.6%) | 0.6224 |
| Statin | 69 (74.19%) | 73 (78.49%) | 0.4901 |
| Indications for surgery | |||
| Asymptomatic | 65 (67.01%) | 65 (66.33%) | >0.999 |
| Symptomatic | 32 (32.99%) | 33 (33.67%) | >0.999 |
| Duplex ultrasound done | 91 (95.79%) | 90 (92.78%) | 0.3702 |
| MRA done | 15 (16.85%) | 11 (11.83%) | 0.3327 |
| CTA done | 32 (34.41%) | 38 (40.43%) | 0.3953 |
| Previous medical history | |||
| Contralateral artery | |||
| Normal | 8 (8.42%) | 13 (13.54%) | 0.0703 |
| <50% | 39 (41.05%) | 51 (53.13%) | |
| 50–70% | 34 (35.79%) | 26 (27.08%) | |
| >70% | 14 (14.74%) | 6 (6.25%) | |
| CABG | 24 (25.26%) | 23 (23.47%) | 0.7716 |
| Operative parameters | |||
| Hemostasis time | 4.90 ± 7.83 (0–73) | 3.09 ± 1.54 (0–12) | 0.0273a |
| Arteriotomy length | 4.21 ± 1.01 (2.2–7) | 4.36 ± 1.05 (1–6.5) | 0.3196 |
| ICA diameter | 5.375 ± 0.8305 (3–8) | 5.3292 ± 0.8058 (3.5–8) | 0.6984 |
| Surgery time (hr) | 2.0959 ± 0.5151 (0.95–3.89) | 2.1613 ± 1.3847 (0.04–11.7) | 0.6698 |
| Perioperative Complications | |||
| TIA | 00 | 00 | NA |
| Stroke | 3 (3.09%)b | 1 (1.02%) | 0.3685 |
| Postcarotid thrombosis | 1 (1.03%) | 1 (1.02%) | >0.999 |
| Infection | 3 (3.09%) | 0 | 0.1212 |
| Reexploration hematoma | 1 (1.03%) | 6 (6.12%) | 0.1183 |
ASA, acetylsalicylic acid; CABG, coronary artery bypass graft; crp, c-reactive protein; ICA, internal carotid artery; NA, not applicable.
Statistically significant at 0.05.
One patient in the ACUSEAL cohort: carotid occlusion, stroke, and death.
Complications
ACUSEAL
Neurologic events
Less than 30 days
Three ipsilateral strokes occurred in the perioperative period. This includes 2 perioperative deaths. One patient was taken back to the operating room in <24 hr for a neurologic deficit and acute carotid thrombosis diagnosed by duplex imaging, who had a known preoperative contralateral occlusion. Despite successful surgical thrombectomy and repair of an intimal flap, the patient had a progressive stroke and death. The second perioperative death was secondary to an ipsilateral hemorrhagic stroke 16 days postoperatively after a fall, while running on a treadmill. He was taking warfarin for chronic atrial fibrillation and he died during readmission. The third patient developed a transient ischemic event during the evening after the CEA. A duplex examination the following morning demonstrated an occlusion of the carotid artery. The attending physician did not reexplore the patient secondary to no remaining focal deficit and uncertainty of the time of occlusion. The patient had an MRI during the hospital readmission that demonstrated multiple ipsilateral infarcts. Two additional patients had readmissions after surgery with severe hypertension and headache, and both underwent CT that was negative for acute stroke. Neither had a new neurologic deficit, and the diagnosis of reperfusion syndrome was made clinically by a neurologist consultant in 1 case and by the operating surgeon in the other.
More than 30 days
Two strokes occurred in the follow-up period. One ipsilateral parietal infarct diagnosed 42 days postoperatively using CT of the head while undergoing workup for confusion, and no permanent deficit was reported. An additional contralateral stroke was reported requiring thrombolytic therapy in the emergency department at 6 months postoperatively and was not counted in further analysis.
Neck hematomas
One patient in the ACUSEAL group underwent surgical exploration for a neck hematoma and was found to have bleeding from the facial vein and underwent surgical ligation of a side branch of that vein. The patient was discharged on postoperative day 2. An additional patient was readmitted for a neck hematoma that was observed for 48 hr and was subsequently discharged without further complications.
Surgical site infections
Three patients had oral antibiotics prescribed for superficial incisional infections within 30 days of surgery. One patient presented with an expanding hematoma 3 months postoperatively and underwent surgical evacuation of the hematoma. Cultures of the hematoma demonstrated methicillin-resistant Staphylococcus aureus on stat Gram stain, and no pseudoaneurysm was reported in the operative note. A vein patch revision was performed at the same time as the neck exploration; however, unfortunately, the patient succumbed to a postoperative myocardial infarction.
Vascu-Guard
Neurologic events
Less than 30 days
One patient in the bovine group on the morning after the CEA had a stroke and recovered completely. Duplex ultrasound revealed carotid thrombosis, and MRI demonstrated multiple ipsilateral punctuate infarcts, and the patient was managed conservatively with no further neurologic event. No other neurologic deficits occurred in the perioperative period.
More than 30 days
Two patients had brainstem infarcts at 6 and 10 months postoperatively by MRI. No ipsilateral strokes occurred.
Neck hematomas
Six patients in the Vascu-Guard arm underwent reexploration for neck hematoma. Findings at the time of exploration included the following, only 1 patient had bleeding from the suture line, the rest were suspected venous bleeding and had no active bleeding source from the patch at reexploration. An additional patient had no significant hematoma but was reexplored for stridor and no hematoma was found.
Surgical site infections
Two patients were reported to have superficial surgical site infections >30 days requiring oral antibiotics and were treated as outpatients without further complicating features.
The overall mean duplex follow-up was 15 months (range: 0.03–43.8 months). The incidence of all ipsilateral strokes (early and late) was 4.17% (3.09% perioperatively) for ACUSEAL vs. 1.02% for bovine patching (P = 0.2091). The perioperative stroke mortality rate for ACUSEAL group was 2.07% and for bovine group was 0% (P = 0.2462). The overall stroke mortality rate was not statistically different for the ACUSEAL patch (8.33%, n = 8) and for the bovine patch (2.04%, n = 2; P = 0.0568). Two patients with ≥70% restenosis were presented in the whole series. The incidence of ≥70% restenosis was 0% for ACUSEAL vs. 2.04% for Vascu-Guard patching (P = 0.621). Both patients with 70% restenosis in bovine patch were men and restenosis was observed at 41.3 and 18.5 months after the surgery respectively.
Table II summarizes the effect of various risk factors on all deaths and strokes (perioperative and late events). None of these factors was statistically significant, except for gender (P = 0.0246), arteriotomy length (P = 0.0428), and stenosis of contralateral artery as past medical history (P = 0.0141). The mean follow-up time was 15.6 months for the bovine pericardial patch and 14.9 months for the ACUSEAL patch (P = 0.679).
Table II.
The effect of risk factors on all deaths and strokes (perioperative and late events)
| Characteristics | No death/stroke (N = 181) | Death/stroke (N = 13) | P value |
|---|---|---|---|
| Mean age | 67.64 | 68.92 | 0.6666 |
| Gender (male) | 95 (52.49%) | 11 (84.62%) | 0.0246a |
| Preoperative laboratories | |||
| Mean total cholesterol | 168.0 | 170.1 | 0.8825 |
| Mean LDL | 86.03 | 99.40 | 0.6423 |
| Mean HDL | 45.71 | 42.50 | 0.5320 |
| Mean triglycerides | 196.9 | 161.6 | 0.5095 |
| Operative parameters | |||
| Mean arteriotomy length | 4.25 | 4.85 | 0.0428a |
| Mean ICA diameter | 5.32 | 5.61 | 0.2238 |
| Comorbidities | |||
| Current smoker | 69 (38.98%) | 8 (61.54%) | 0.1099 |
| Past smoker | 82 (47.13%) | 3 (25.00%) | 0.1367 |
| Diabetes mellitus | 66 (36.46%) | 5 (38.46%) | >0.999 |
| Congestive heart failure | 15 (8.29%) | 1 (7.69%) | >0.999 |
| Peripheral artery disease | 58 (32.04%) | 4 (30.77%) | >0.999 |
| Hypertension | 154 (85.08%) | 10 (76.92%) | 0.4284 |
| Hypercholesterolemia | 147 (81.22%) | 10 (76.92%) | 0.7163 |
| Indications for surgery | |||
| Asymptomatic | 122 (67.40%) | 8 (61.54%) | 0.7617 |
| Symptomatic | 59 (32.60%) | 5 (38.46%) | 0.7617 |
| Previous medical history | |||
| Contralateral artery | |||
| Normal | 18 (10.17%) | 3 (23.08%) | 0.0141a |
| <50% | 85 (48.02%) | 5 (38.46%) | |
| 50–70% | 59 (33.33%) | 1 (7.69%) | |
| >70% | 15 (8.47%) | 4 (30.77%) | |
| CEA on operated site | 1 (0.56%) | 0 | >0.999 |
| CABG | 45 (25.00%) | 2 (15.38%) | 0.7380 |
CABG, coronary artery bypass graft.
Statistical Significant at P < 0.05.
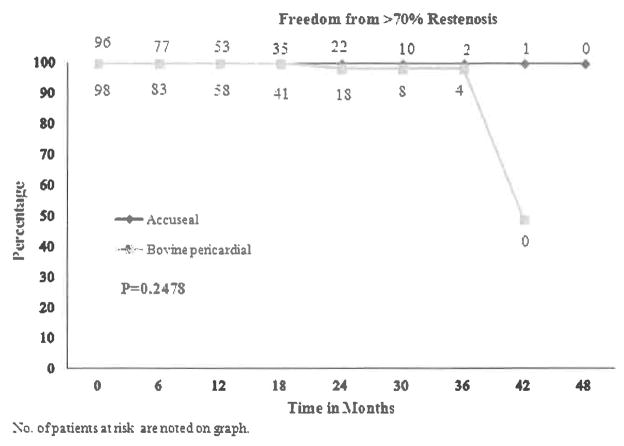
The freedom from ≥70% restenosis was 100% for ACUSEAL patching vs. 100%, 98%, and 98%, at 1, 2, and 3 years for bovine patching (P = 0.2478, Fig. 1). The cumulative stroke-free rates at 1, 2, and 3 years were 96% for ACUSEAL patching vs. 99% for bovine patching, respectively (P = 0.17, Fig. 2). There were only 2 study-related deaths due to stroke, both with the ACUSEAL patch.
Fig. 1.
Freedom from >70% restenosis.
Fig. 2.
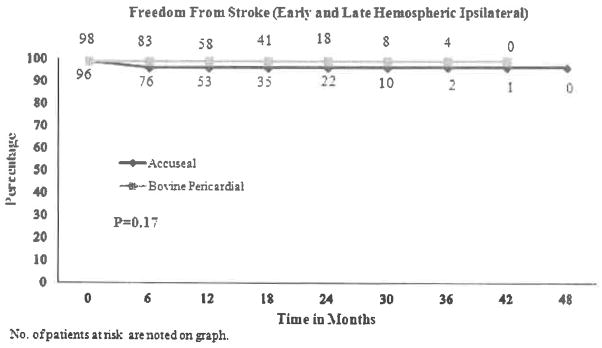
Freedom from stroke (early and late hemispheric ipsilateral).
DISCUSSION
Over the past 20 years at our institution, many studies have been performed evaluating the results of endarterectomy. The initial work began in 1996, comparing primary closure to autologous and prosthetic patches. Subsequently, long-term follow-up from this study was published 2 years later in the Journal of Vascular Surgery.15
With multiple trials and the initial study at our institution, showing no statistical benefit of using autologous patch material versus prosthetic patching, the ideal patch became a topic of investigation over the next 2 decades. An obvious choice was to compare the use of collagen-impregnated Dacron to polytetrafluorethylene (PTFE) patches because both materials were commonly being used by vascular surgeons, Dacron predominately due to its hemostatic properties. The short-term results of this prospective trial questioned the thrombogenicity of knitted Dacron patching (Hemashield; Boston Scientific, Oakland, NJ), and the long-term results of this prospective study of 200 carotid arteries demonstrated a higher rate of severe recurrent stenosis, compared with standard PTFE (Gore-tex; W.L. Gore & Associates) at 15% vs. 0%, at a mean follow-up of 26 months. Carotid artery occlusion was reported in 6% of those with standard Hemashield vs. 0% with PTFE patching.16,17
Although, results from our single institution prospective randomized trial described previously demonstrated superior results, suture line bleeding was prolonged in comparison to Dacron patching. The use of CV-6 PTFE sutures with a 1:1 needle-to-suture ratio improved suture line bleeding. However, widespread use of this patch was not seen, even after the results from our study. W.L Gore subsequently released a modified PTFE (ACUSEAL) that had a shorter hemostasis time (mean: 3 min) compared with that of standard wall PTFE, despite using standard 6-0 prolene with a BV-1 needle. In addition, we demonstrated that only 1 % of neck hematomas required surgery in a prospective non-randomized study with 200 carotid arteries. The freedom from >70% restenosis was 94% at 4 years.18
Subsequently, our group evaluated the next generation PTFE (ACUSEAL) versus a proposed less-thrombogenic Dacron patch (Finesse; Hemashield; Boston Scientific). In a prospective randomized fashion, 200 carotid arteries were compared with 100 in each cohort with short- and long-term follow-up. The perioperative results were similar with respect to neurologic events, and a clinically nonsignificant but statistically longer hemostasis time, with the ACUSEAL patch at 5.1 vs. 3.7 min. However, long-term freedom from recurrent stenosis was superior for the ACUSEAL patch at 3 years, 89% vs. 79%, respectively (P = 0.04).
The use of bovine pericardium for vascular applications has escalated at our institution and others. The short- and long-term results over the past decade have been excellent,20 with the largest experience reported by Ladowski et al. with more than 800 endarterectomies and a 5-year restenosis rate of 7%.20 Despite the widespread use of this patch, only 1 small prospective trial has been conducted with 52 patients where bovine pericardium patches were compared with Dacron.21
In this present study, we conducted a prospective randomized trial comparing the bovine pericardial patch versus the ACUSEAL patch. This study directly compares these 2 patches. In addition, a detailed literature review of all available studies using either PTFE or bovine was constructed for reference (Table III). This study provides prospective data with early results using both the ACUSEAL and Vascu-Guard patches. Although the follow-up is not as long as most of the retrospective data available in the literature, the accuracy of prospectively collected and institutional review board–scrutinized outcomes cannot be underscored enough. The results suggest that physician’s preference, such as handling, can be used between these 2 patches with comparable results. Although neck hematomas requiring reexploration occurred more frequently in the Vascu-Guard cohort, only 1 patient had bleeding noted at the suture line at the time of reexploration. The remaining patients had suspected venous bleeding and may be a result of simple random occurrence rather than patch-related issues.
Table III.
Literature review of polytetrafluorethylene (PTFE) and bovine pericardium patches
| Author | Number of CEA | 30-day stroke (%) | Mean F/U | Recurrent stenosis |
|---|---|---|---|---|
| PTFE | ||||
| AbuRahma et al.17,a | 100 | 2 | 21 | >70% = 11% at 36 months |
| Grego et al.22,a | 80 | 1 | 45 | >50% = 9.8% at 30 months |
| AbuRahma et al.18 | 200 | 1 | 21 | >70% = 6% at 48 months |
| Gonzalez-Fajardo et al.23,a | 50 | 2 | 29 | >50% = 4% at NA |
| Katz et al.4,a | 49 | 2 | 29 | >50% = 0% at 24 months |
| Lord et al.24,a | 47 | 2 | NA | NA |
| AbuRahma et al.15,a | 134 | 1 | 30 | >50% = 2% at 36 months |
| AbuRahma et al.19,a | 100 | 0 | 26 | >70% = 0% at 36 months |
| Bovine | ||||
| Hines et al.25 | 61 | 0 | 13 | >50% = 4.4% at 12 months |
| Grimsley et al.26 | 129 | 0 | 41 | >70% = 2% at 41 months |
| Ho et al.27 | 457 | 2.2 | 46 | >75% = 1 % at 60 months |
| Ladowski et al.20 | 845 | <1 | 19 | >60% = 7.4% at 13 months |
| Kim et al.28 | 252 | 2 | 62 | >50% = 2.8% at 62 months |
| Marien et al.21,a | 51 | 2 | NA | >60% = 5% at NA |
| Bisdas et al.29 | 143 | 2.1 | 1 | NA |
| Biasi et al.30 | 323 | 1.5 | NA | >60% = 10% at 60 months |
| Matsagas et al.31 | 148 | 1.4 | 20 | >50% = 2% at 36 months |
| Neuhauser et al.32 | 59 | 0 | 12 | >70% = 1.6% at 18 months |
Mean F/u = follow-up in months.
Randomized trials.
The most important early surrogate of a cardiovascular patch’s performance is the thrombogenic potential of the patch. Our randomized results mirror those from other prospective and retrospective reviews, with <3% perioperative events noted in all series (Table III). Operative hemostasis also affects surgeon preference; most surgeons in this study said that the PTFE takes longer to achieve operative hemostasis, but required <2 min longer than the bovine patch, which did not affect operative time.
The number of patients taking preoperative clopidogrel and warfarin was not statistically different between the 2 groups. Although we had a standardized practice for antiplatelet management perioperatively that included continuing aspirin and holding clopidogrel for 7 days before the operation, we did not routinely check platelet assays of bleeding times to assess preoperative platelet activity. Although there were more neck explorations in the bovine group, only 1 patient clearly had bleeding from needle holes. The other patients had suspected venous oozing, but this could have been oozing from the patch that had stopped by the time exploration was performed.
The cost of a particular patch can play a role in choosing a patch. At our institution, the acquisition cost is <100 US dollars between patches. The best surrogate of long-term results of specific patching is the incidence of recurrent stenosis and neurologic events. Unfortunately, the results of this trial do not provide a follow-up that is long enough to determine the risk of recurrent stenosis in the long-term and secondary to follow-up period limitations. Our group’s practice has not changed dramatically after this trial, with those continuing to use their preferred patch based on handling characteristics as before the study.
In conclusion, the results of this first prospective randomized study showed that both patches for CEA were comparable in early outcomes.
Footnotes
Presented at the 27th Annual Meeting of the Eastern Vascular Society, The Greenbrier, White Sulphur Springs, WV, September 19–22, 2013.
References
- 1.Archie JP., Jr Prospective randomized trials of carotid endarterectomy with primary closure and patch reconstruction: the problem is power. J Vasc Surg. 1997;25:1118–20. doi: 10.1016/s0741-5214(97)70137-x. [DOI] [PubMed] [Google Scholar]
- 2.AbuRahma AF, Khan JH, Robinson PA, et al. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: perioperative (30-day) results. J Vasc Surg. 1996;24:998–1006. doi: 10.1016/s0741-5214(96)70045-9. discussion 1006–1007. [DOI] [PubMed] [Google Scholar]
- 3.AbuRahma AF, Stone PA, Deem S, et al. Proposed duplex velocity criteria for carotid restenosis following carotid endarterectomy with patch closure. J Vasc Surg. 2009;50:286–91. doi: 10.1016/j.jvs.2009.01.065. [DOI] [PubMed] [Google Scholar]
- 4.Katz D, Snyder SO, Gandhi RH, et al. Long-term follow-up for recurrent stenosis: a prospective randomized study of expanded polytetrafluoroethylene patch angioplasty versus primary closure after carotid endarterectomy. J Vasc Surg. 1994;19:198–203. doi: 10.1016/s0741-5214(94)70095-8. discussion 204–195. [DOI] [PubMed] [Google Scholar]
- 5.Ranaboldo CJ, Barros D’Sa AA, Bell PR, et al. Randomized controlled trial of patch angioplasty for carotid endarterectomy. The Joint Vascular Research Group. Br J Surg. 1993;80:1528–30. doi: 10.1002/bjs.1800801211. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal D, Archie JP, Jr, Garcia-Rinaldi R, et al. Carotid patch angioplasty: immediate and long-term results. J Vasc Surg. 1990;12:326–33. [PubMed] [Google Scholar]
- 7.Eikelboom BC, Ackerstaff RG. Patch grafting in carotid endarterectomy. Stroke. 1989;20:1288–9. [PubMed] [Google Scholar]
- 8.Clagett GP, Patterson CB, Fisher DF, Jr, et al. Vein patch versus primary closure for carotid endarterectomy. A randomized prospective study in a selected group of patients. J Vasc Surg. 1989;9:213–23. doi: 10.1067/mva.1989.vs0090213. [DOI] [PubMed] [Google Scholar]
- 9.Imparato AM. The role of patch angioplasty after carotid endarterectomy. J Vasc Surg. 1988;7:715–6. [PubMed] [Google Scholar]
- 10.Hertzer NR, Beven EG, O’Hara PJ, et al. A prospective study of vein patch angioplasty during carotid endarterectomy. Three-year results for 801 patients and 917 operations. Ann Surg. 1987;206:628–35. doi: 10.1097/00000658-198711000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archie JP., Jr Prevention of early restenosis and thrombosis-occlusion after carotid endarterectomy by saphenous vein patch angioplasty. Stroke. 1986;17:901–5. doi: 10.1161/01.str.17.5.901. [DOI] [PubMed] [Google Scholar]
- 12.Economopoulos KJ, Gentile AT, Berman SS. Comparison of carotid endarterectomy using primary closure, patch closure, and eversion techniques. Am J Surg. 1999;178:505–10. doi: 10.1016/s0002-9610(99)00247-0. [DOI] [PubMed] [Google Scholar]
- 13.Ballotta E, Da Giau G, Saladini M, et al. Carotid endarterectomy with patch closure versus carotid eversion endarterectomy and reimplantation: a prospective randomized study. Surgery. 1999;125:271–9. [PubMed] [Google Scholar]
- 14.Baker JD, Rutherford RB, Bernstein EF, et al. Suggested standards for reports dealing with cerebrovascular disease. J Vasc Surg. 1988;8:721–9. 478. doi: 10.1067/mva.1988.avs0080721. [DOI] [PubMed] [Google Scholar]
- 15.AbuRahma AF, Robinson PA, Saiedy S, et al. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: long-term follow-up. J Vasc Surg. 1998;27:222–32. doi: 10.1016/s0741-5214(98)70353-2. discussion 233–224. [DOI] [PubMed] [Google Scholar]
- 16.AbuRahma AF, Robinson PA, Hannay RS, et al. Prospective controlled study of carotid endarterectomy with hemashield patch: is it thrombogenic? J Vasc Surg. 2001;35:167–74. doi: 10.1177/153857440103500302. [DOI] [PubMed] [Google Scholar]
- 17.AbuRahma AF, Hopkins ES, Robinson PA, et al. Prospective randomized trial of carotid endarterectomy with polytetrafluoroethylene vs. collagen-impregnated Dacron (Hemashield) patching: late follow up. Ann Surg. 2002;237:885–93. doi: 10.1097/01.SLA.0000067741.10420.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AbuRahma AF, Stone PA, Welch CA, et al. Prospective study of carotid endarterectomy with modified polytetrafluoroethylene (ACUSEAL) patching: early and late results. J Vasc Surg. 2005;41:789–93. doi: 10.1016/j.jvs.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 19.AbuRahma AF, Stone PA, Elmore M, et al. Prospective randomized trial of ACUSEAL (Gore-Tex) vs. Finesse (Hemashield) patching during carotid endarterectomy: long-term outcome. J Vasc Surg. 2008 Apr 14;48:99–103. doi: 10.1016/j.jvs.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 20.Ladowski JM, Ladowski JS. Retrospective analysis of bovine pericardium (Vascu-Guard) for patch closure in carotid endarterectomies. Ann Vasc Surg. 2011;25:646–50. doi: 10.1016/j.avsg.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Marien BJ, Raffeto JD, Seidman CS, et al. Bovine pericardium vs. Dacron for patch angioplasty after carotid endarterectomy. Arch Surg. 2002;137:785–8. doi: 10.1001/archsurg.137.7.785. [DOI] [PubMed] [Google Scholar]
- 22.Grego F, Antonello M, Lepidi S, et al. Prospective, randomized study of external jugular vein patch vs. polytetrafluoroethylene patch during carotid endarterectomy: perioperative and long term results. J Vasc Surg. 2003;38:1232–40. doi: 10.1016/s0741-5214(03)00912-1. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Fajardo JA, Perez JL, Mateo AM. Saphenous vein patch vs. polytetrafluoroethylene patch after carotid endarterectomy. J Cardiovasc Surg (Torino) 1994 Dec;35:523–8. [PubMed] [Google Scholar]
- 24.Lord RSA, Raj TB, Stary DL, et al. Comparison of saphenous vein patch, polytetrafluoroethylene patch, and direct arteriotomy closure after carotid endarterectomy. J Vasc Surg. 1989;9:521–9. [PubMed] [Google Scholar]
- 25.Hines GL, Feuerman M, Cappello D, et al. Results of carotid endarterectomy with pericardial patch angioplasty: rate and predictors of restenosis. Ann Vasc Surg. 2007;21:767–71. doi: 10.1016/j.avsg.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Grimsley BR, Wells JK, Pearl GJ, et al. Bovine pericardial patch angioplasty in carotid endarterectomy. Am Surg. 2001;67:890–5. [PubMed] [Google Scholar]
- 27.Ho KJ, Nguyen LL, Menard MT. Intermediate-term outcome of carotid endarterectomy with bovine pericardial patch closure compared with Dacron patch and primary closure. J Vasc Surg. 2012;55:708–14. doi: 10.1016/j.jvs.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Cho YP, Kwon TW, et al. Ten-year comparative analysis of bovine pericardium and autologous vein for patch angioplasty in patients undergoing carotid endarterectomy. Ann Vasc Surg. 2012;26:353–8. doi: 10.1016/j.avsg.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Bisdas T, Pichlmaier M, Bisdas S, et al. Early neurologic outcome after bovine pericardial patch angioplasty in 599 patients undergoing carotid endarterectomy. Vascular. 2010;18:147–53. doi: 10.2310/6670.2010.00022. [DOI] [PubMed] [Google Scholar]
- 30.Biasi GM, Sternjakob S, Mingazzini PM, et al. Nine-year experience of bovine pericardium patch angioplasty during carotid endarterectomy. J Vasc Surg. 2002;36:271–7. doi: 10.1067/mva.2002.123685. [DOI] [PubMed] [Google Scholar]
- 31.Matsagas MI, Bali C, Arnaoutoglou E, et al. Carotid endarterectomy with bovine pericardium patch angioplasty. Mid-term results. Ann Vasc Surg. 2006;20:614–9. doi: 10.1007/s10016-006-9102-3. [DOI] [PubMed] [Google Scholar]
- 32.Neuhauser B, Oldenburg WA. Polyester vs. bovine pericardial patching during carotid endarterectomy: early neurologic events and incidence of restenosis. Cardiovasc Surg. 2003;11:465–70. doi: 10.1016/S0967-2109(03)00109-1. [DOI] [PubMed] [Google Scholar]