Figure 2.
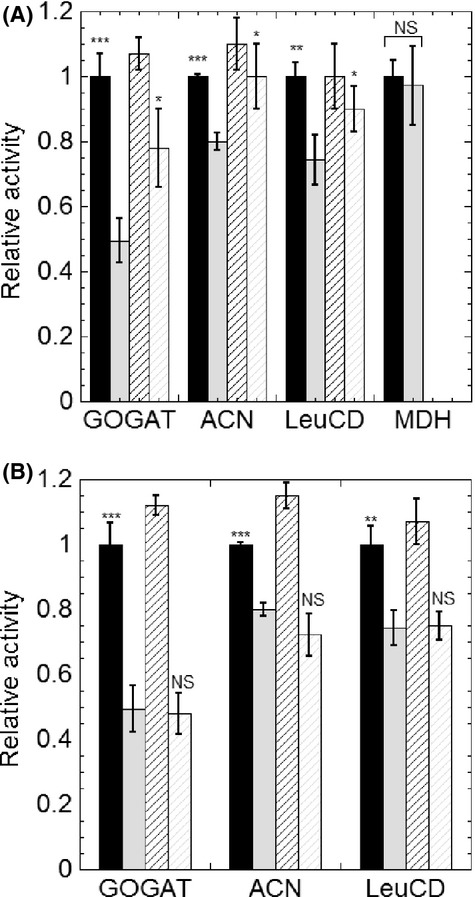
Lack of BSH leads to lower activity levels of Fe–S enzymes. (A) Activities of the Fe–S proteins glutamate synthase (GOGAT), aconitase (ACN), isopropyl malate isomerase (LeuCD) and the non-Fe–S protein malate dehydrogenase (MDH) in cell lysates of Bacillus subtilis wild type (black) and ΔbshA strain (gray) cultured in MM (filled bar) or MM containing an additional 50 μmol/L of Fe2+ (diagonal striped bar). (B) Bacillus subtilis wild-type strain (black) and ΔbshA strain (gray) cultured MM and the activity of Fe–S enzymes was quantified before (filled bar) and after the addition of 50 μmol/L of Fe2+ to cell lysates (upright striped bar). The activities of GOGAT, ACN, LeuCD, and MDH for wild-type B. subtilis were measured to be 40, 131, 17, and 1051 (nmol/min per mg total protein in crude extract), respectively. The activities of the ΔbshA strain were normalized to the activities of wild type in MM. All assays were performed in triplicates. The statistical analysis was performed using unpaired t test, P values were obtained by comparing the ΔbshA without iron to wild-type w/o Fe and to ΔbshA with Fe. Comparisons of wild-type activities with and without Fe in both panels were not statistical significant. (NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001).
