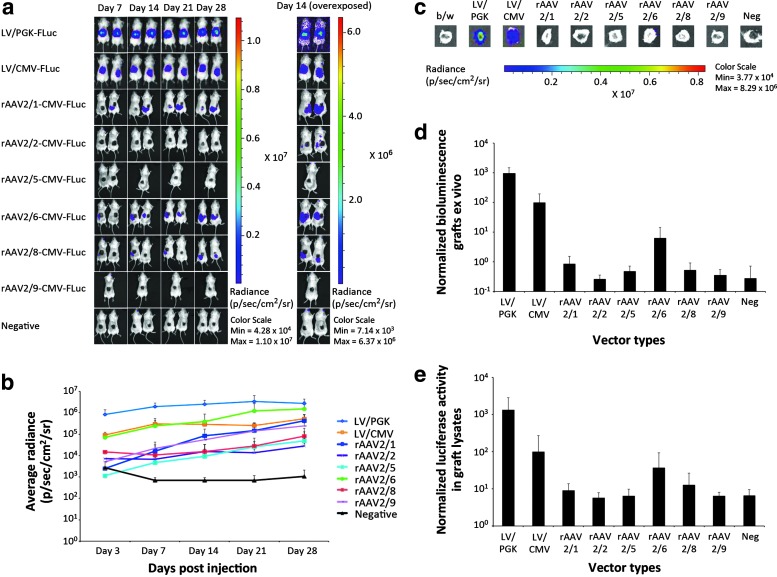
Figure 2.
In vivo and ex vivo detection of luciferase after intradermal injections of LV or rAAV2 serotype 1, 2, 5, 6, 8, or 9 into xenografted human skin. (a) In vivo imaging of luciferase activity after intradermal vector administration. Shown are representative images from days 7, 14, 21, and 28 of xenotransplanted mice in groups injected intradermally with LV/PGK-FLuc, LV/CMV-FLuc, rAAV2/1-CMV-FLuc, rAAV2/2-CMV-FLuc, rAAV2/5-CMV-FLuc, rAAV2/6-CMV-FLuc, rAAV2/8-CMV-FLuc, rAAV2/9-CMV-FLuc, or negative controls. (b) Bioluminescence from injected xenografted human skin was recorded on days 3, 7, 14, 21, and 28 and the results are based on the signal intensity computed from a standardized region of interest. The experiment was performed twice, and data for each group were pooled from the two experiments (LV, rAAV2/1, rAAV2/2, rAAV2/6, rAAV2/8, and negative controls, n = 4; rAAV2/5 and rAAV2/9, n = 3). (c) Ex vivo detection of luciferase activity in human skin xenografts exposed to LV or rAAV2 serotype 1, 2, 5, 6, 8, or 9. Shown are representative images of the human skin xenografts after excision of these immediately after the mice were killed on day 28. (d) Depiction of average radiance after normalization to 102 for LV/CMV-FLuc in the human skin xenografts ex vivo on day 28 (n = 3). (e) Bioluminescence intensity depicted as the average luciferase activity in lysates of the excised human skin grafts normalized to total protein concentration (n = 3). LV, lentiviral vector; rAAV, recombinant adeno-associated viral vectors; p/sec/cm2/sr, photons per second per centimeter squared per steradian. Data are presented as means + SD.