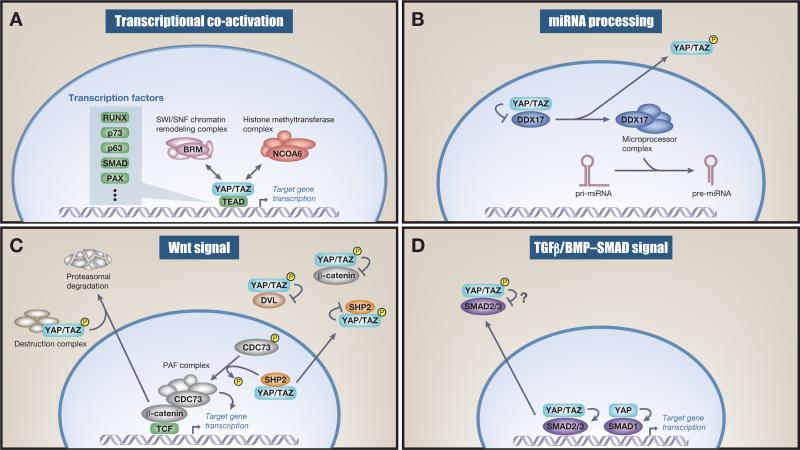
Figure 3.
Function of nuclear and cytoplasmic Yes-associated protein (YAP) and transcriptional coactivator with a PDZ-binding domain (TAZ). (A) Transcriptional coactivation. Nuclear YAP and TAZ bind to transcription factors (TFs) and recruit the NCOA6 methyltransferase complex as well as the BRM-containing SWI/SNF chromatin-remodeling complex, which mediates TF target-specific gene transcription. (B) miRNA processing. Nuclear YAP inhibits DDX17, a regulatory component of the microprocessor complex. This nuclear inhibition decreases global miRNA on YAP stimulation. Of note, some specific miRNAs are upregulated on YAP nuclear activation. (C) Wnt signaling. Cytoplasmic YAP and TAZ sequester β-catenin and in some instances recruit β-TrCP to the destruction complex. This leads to nuclear β-catenin downregulation and therefore dampens Wnt signaling. Cytoplasmic YAP and TAZ also negatively regulate Wnt signaling by sequestering β-catenin and disheveled (DVL). YAP and TAZ dictate nuclear and cytoplasmic localization of SHP2 and therefore its function. This feeds into the Wnt signaling pathway because nuclear SHP2 dephosphorylates parafibromin (CDC73), which leads to increased β-catenin transcriptional activity. (D) Transforming growth factor beta (TGFβ)/BMP–SMAD signal. Cytoplasmic YAP and TAZ sequester SMAD2/3 in the cytoplasm and therefore inhibit SMAD2/3-mediated signaling, although these mechanisms seem not to be universal and somewhat controversial. Nuclear YAP activates SMAD1-mediated transcription.