Scheme 1.
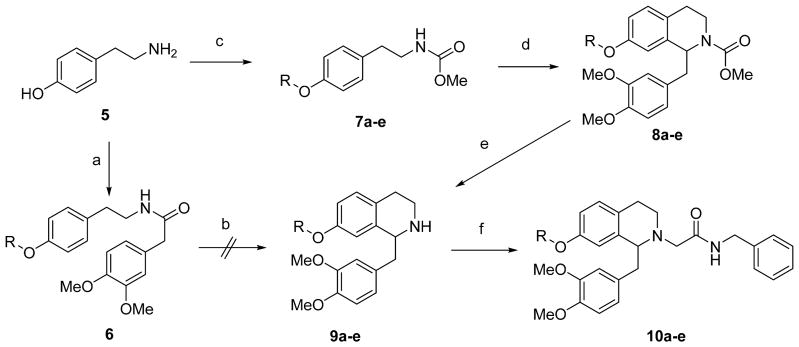
Synthesis of 7-alkoxytetrahydroisoquinoline derivatives
Reagents and Conditions: (a) (i) 3,4-Dimethoxyphenylacetic acid, BOP, iPr2EtN, DMF; (ii) R-I or R-Br, K2CO3, DMF; (b) (i) POCl3, toluene; (ii) NaBH4, MeOH; (c) (i) MeOCOCl, iPr2EtN, CH2Cl2; (ii) R-I or R-Br, K2CO3, DMF; (d) 3,4-dimethoxyphenylacetaldehyde, TFA; (e) KOH, NH2NH2.H2O, (CH2OH)2; (f) BrCH2CONHBn, iPr2EtN, Bu4NI, DMF.
