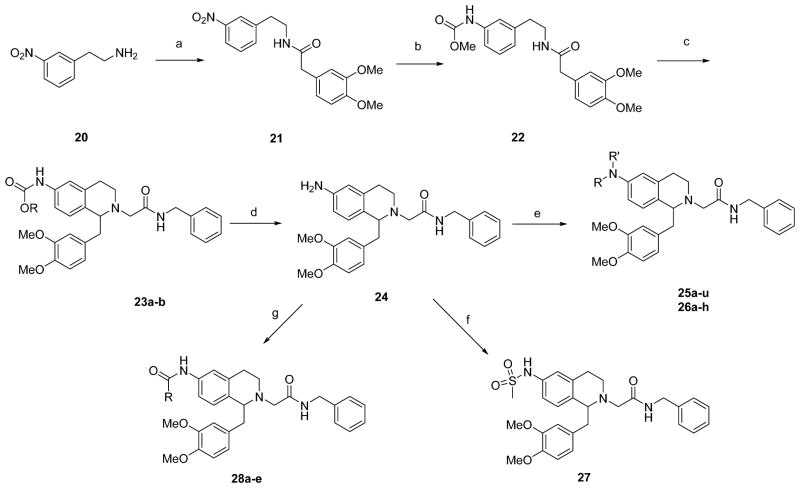
Scheme 3.
Synthesis of 6-aminotetrahydroisoquinoline derivatives.
Reactions and Conditions: (a) 3,4-dimethoxyphenylacetic acid, BOP, iPr2EtN, DMF; (b) (i) Raney Ni, NH2NH2.H2O, EtOH; (ii) MeOCOCl, iPr2EtN, CH2Cl2; (c) (i) POCl3, toluene; (ii) NaBH4, MeOH; (iii) BrCH2CONHBn, iPr2EtN, Bu4NI, DMF; (d) 2N NaOH (aq), MeOH; (e) aldehyde, Na(OAc)3BH, 1–2-DCE or alkyl halide, iPr2EtN, DMF; (f) (i) MsCl, Et3N, CH2Cl2; (ii) 2N NaOH (aq); (g) RCO2H, BOP, iPr2EtN, CH2Cl2 or RCOCl/(RCO)2O, iPr2EtN, CH2Cl2 or isocyanate, toluene.