Table 3.
6-Amino tetrahydroisoquinoline orexin antagonists.
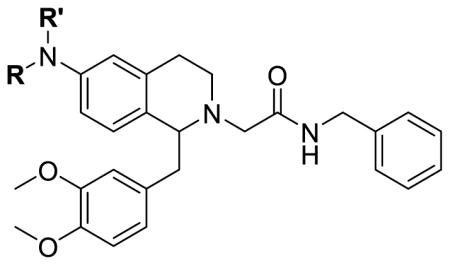
| ||||
|---|---|---|---|---|
| No. | R | R′ | OX1 Ke (nM)a | OX2 Ke (nM)a |
| 25a | Methyl | Methyl | 1370 ± 100 | b |
| 25b | n-Propyl | H | >10000 | b |
| 25c | n-Propyl | Methyl | 619 ± 96 | b |
| 25d | n-Propyl | n-Propyl | 2350 ± 490 | b |
| 25e | iso-Propyl | H | >10000 | b |
| 25f | Cyclopropylmethyl | H | >10000 | b |
| 25g | Cyclopropylmethyl | Methyl | 1400 ± 240 | b |
| 25h | Benzyl | H | 1100 ± 290 | b |
| 25i | Benzyl | Methyl | >10000 | b |
| 25j |
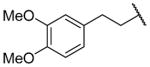
|
H | 2180 ± 360c | b |
| 25k | 3-Phenylpropyl | H | 1180 ± 80 | b |
| 25l | 4-Phenoxybutyl | H | 2080 ± 20 | b |
| 25m | 4-Pyridylmethyl | H | >10,000 | b |
| 25n | 3-Pyridylmethyl | H | 1270 ± 110 | b |
| 25o |
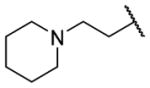
|
H | >10000 | b |
| 25p | Pyrrolidine | 2480 ± 6 | b | |
| 25q | Piperidine | >10000 | b | |
| 25r | n-Pentyl | H | >10000 | b |
| 25s | n-Pentyl | Methyl | >10000 | b |
| 25t | n-Hexyl | H | >10000 | b |
| 25u | n-Hexyl | Methyl | >10000 | b |
| 26a |
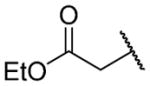
|
H | 427 ± 69 | b |
| 26b |
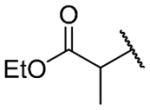
|
H | >10000 | b |
| 26c |
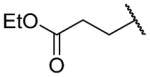
|
H | 809 ± 200 | b |
| 26d |
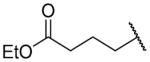
|
H | 591 ± 67 | >10000 |
| 26e |
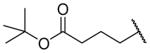
|
H | 1010 ± 200d | b |
| 26f |
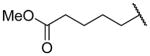
|
H | 1600 ± 190 | b |
| 26g |
|
H | >10000 | b |
| 26h |
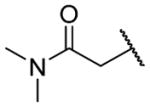
|
H | >10000 | b |
| 27 | Methylsulfanyl | H | >10000 | b |
| 28a | Acetyl | H | >10000 | b |
| 28b | Hexanoyl | H | >10000 | >10000 |
| 28c | Benzoyl | H | >10000 | b |
| 23a | Methyl carbamoyl | H | 2400 ± 320 | >10000 |
| 23b | Ethyl carbamoyl | H | >10000 | >10000 |
| 28d | PhNHCO | H | >10000 | b |
| 28e | n-Hexyl-NHCO | H | 5230 ± 680 | b |
Ke values are the mean ± SEM of three independent experiments performed in duplicate. Results where Ke > 10 μM are N=2.
Less than 50% inhibition at 10 μM.
Pre-incubation of antagonist and receptor was 45 min.
Pre-incubation of antagonist and receptor was 1 hr.
