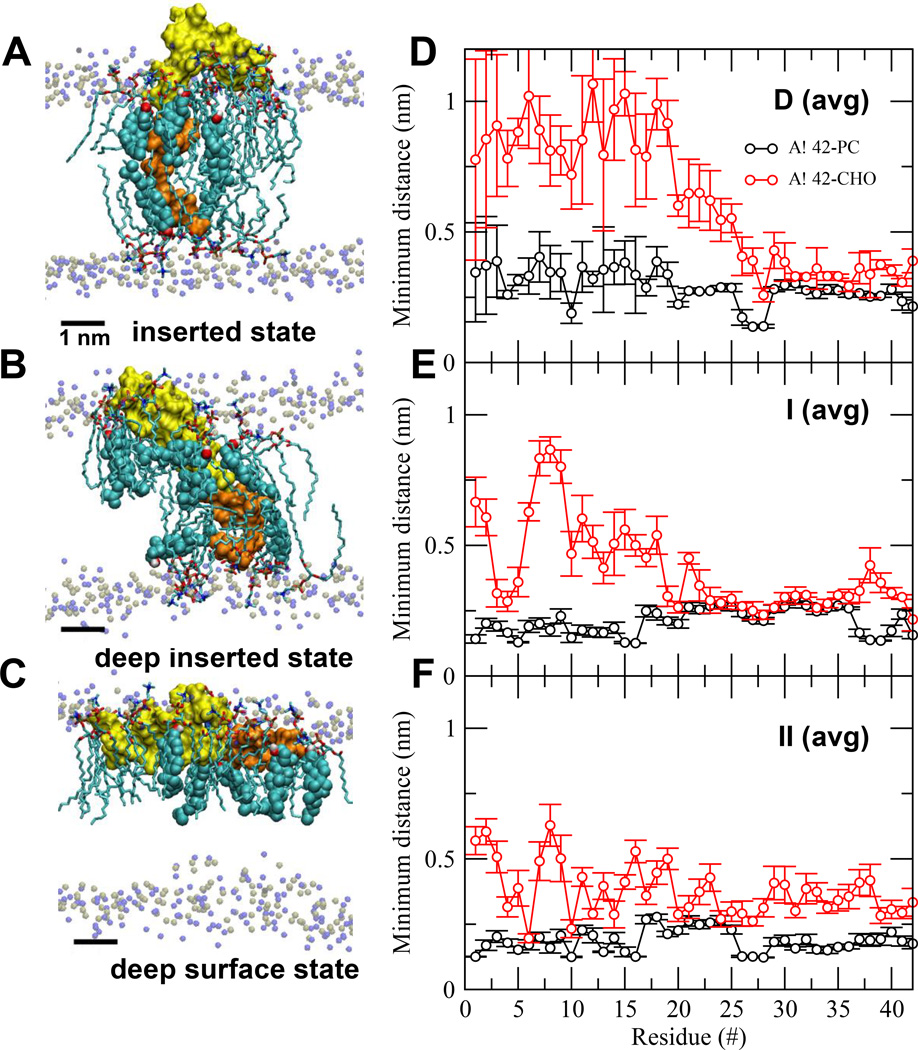
FIG. 3. Protein orientational states and lipid/protein interactions in Aβ42/lipid/water/ion complexes.
UA structures of inserted (A), deep inserted (B) and deep surface (C) states of Aβ42 in Aβ42/lipid/water/ion complexes. D1-N27 or non-LID (yellow) and K28-A42 or LID (orange) segments of the protein are rendered in surface representation. The polar phosphate (silver) and NC3 (blue) of all PC are shown. The annular (AL) lipids, AL-CHO (color spheres) and AL-PC (sticks), are shown. The average (avg) minimum distance between Aβ42 and CHO or PC across all repeated replicates for the last 50 ns as a function of residue position of Aβ42 in D (avg) (panel D), I (avg) (panel E) and II (avg) (panel F) are shown. The bars indicate standard errors. See Materials and Methods for details in averaging.