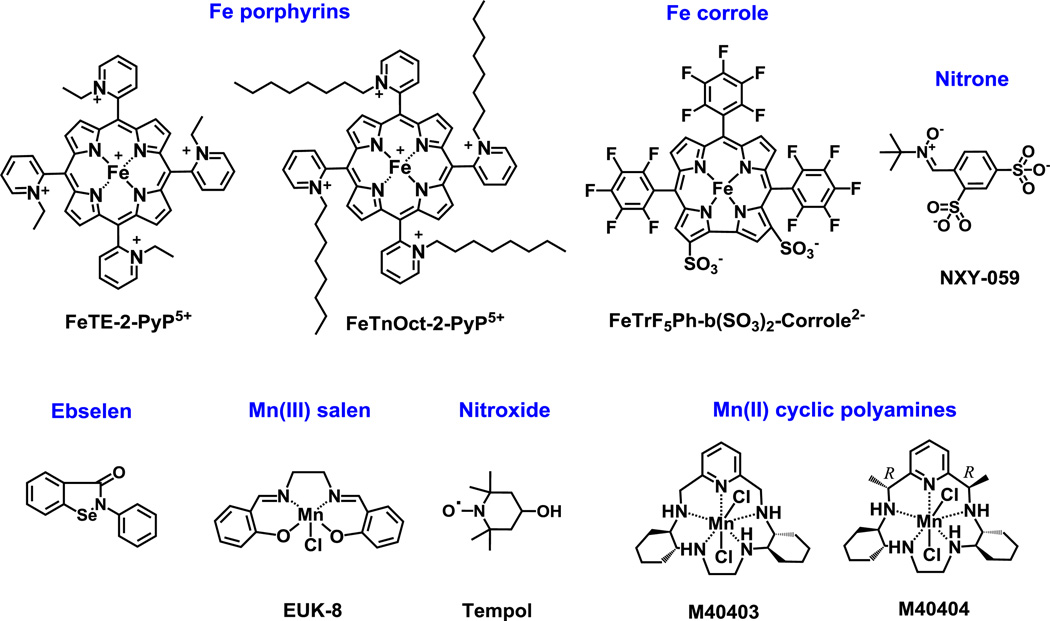
Figure 2. Structures of other redox-active drugs whose catalase-like activity was assessed herein.
Compounds include SOD mimics of different magnitudes of SOD-like activities, such as Fe porphyrins, as well as compounds such as nitrones and nitroxides which are not SOD mimics but can cycle with other reactive species whereby eventually removing O2.− also [19]. Nitrone can trap free radicals such as O2.− and form nitroxide and thus affect O2.− levels. Nitroxide in turn can be oxidized with CO3.− to oxoammonium cation which then rapidly oxidizes O2.− closing the catalytic cycle. Mn(II) cyclic polyamine, M40403 is a very potent SOD mimic, but its SOD-inactive analog, M40404, is not. If Mn complexes fall apart they would release Mn. Moreover Mn(II) low molecular weight complexes, and in particular Mn(II) lactate, are SOD mimics also [19, 21]. The kinetic and thermodynamic data on these compounds are listed in Tables 2 and 3. The data for Fe corrole are taken from literature [19, 21, 52, 53].