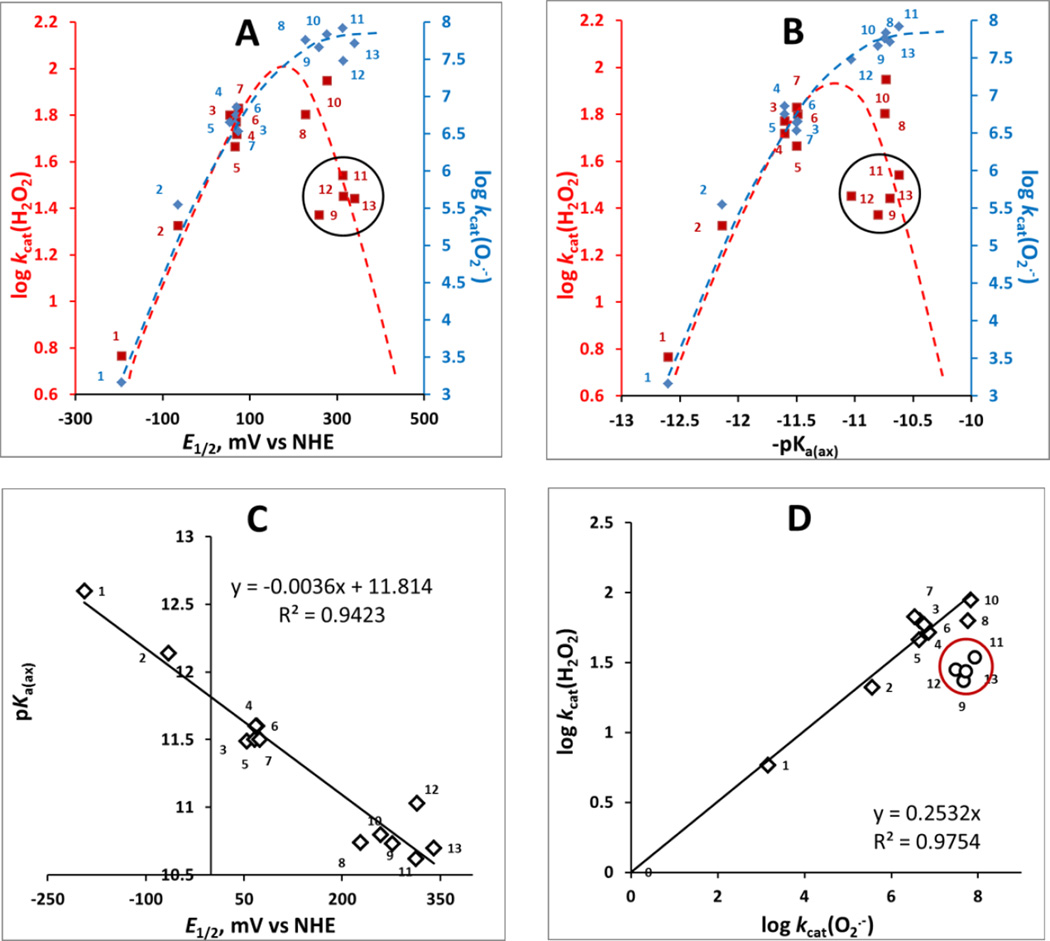
Figure 4. The relation between the log kcat(H2O2) and log kcat(O2.−) each with E1/2 of MnIIIP/MnIIIP redox couple in mV vs NHE (A) and with proton dissociation constant of axial water, pKa(ax) (B); the relation between the pKa(ax) and E1/2 for MnIIIP/MnIIP redox couple (C); and the relationship between the log kcat(H2O2) and log kcat(O2.−) (D).
The ability of MnP to catalyze H2O2 dismutation depends on H2O2 binding in a 1st step of dismutation process and thus on the electron-deficiency of the metal site which is described with pKa(ax) (B). The E1/2 for any couple that involves species in high-oxidation states (eqs [3] and [4]) are similar for all Mn porphyrins (see under Catalysis of H2O2 dismutation by Mn porphyrins); thus the 2nd step, electron transfer, has no impact on the magnitude of kcat(H2O2). In turn, the pKa(ax) was reported and confirmed here with new MnPs [49] to parallel the E1/2 of MnIIIP/MnIIP redox couple (C) [50, 68]. Consequently, the log kcat(H2O2) paralells the E1/2 of MnIIIP/MnIIP redox couple (A) though this couple is not involved in H2O2 dismutation. Since the E1/2 of MnIIIP/MnIIP redox couple controls the catalysis of O2.− dismutation also, the log kcat(H2O2) is proportional to log kcat(O2.−) (D). Please note that in plots A and B blue rhombs relate to O2.− dismutation while red squares relate to H2O2 dismuation. To help identify MnPs on plots please consult also Table 1. We have drawn dashed lines to indicate different trends in the catalysis of O2.− (blue) and H2O2 dismutations by MnPs (red). Please note that with a predominantly outer-sphere electron transfer in catalysis of O2.− dismutation (which involves electron hoping and not O2.− binding) we have a different behavior when compared to the catalysis of H2O2 dismutation by MnPs; the latter involves binding of H2O2 to the metal site (plots A and B). In such scenario, steric hindrance, imposed by longer alkyl chains with and without polar oxygen atoms within chains, plays significant role. Encircled are the MnPs with long and bulky ortho N-pyridyl substituents: MnTnOct-2-PyP5+, MnTnHex-2-PyP5+, MnTnHexOE-2-PyP5+, MnTPhE-2-PyP5+.