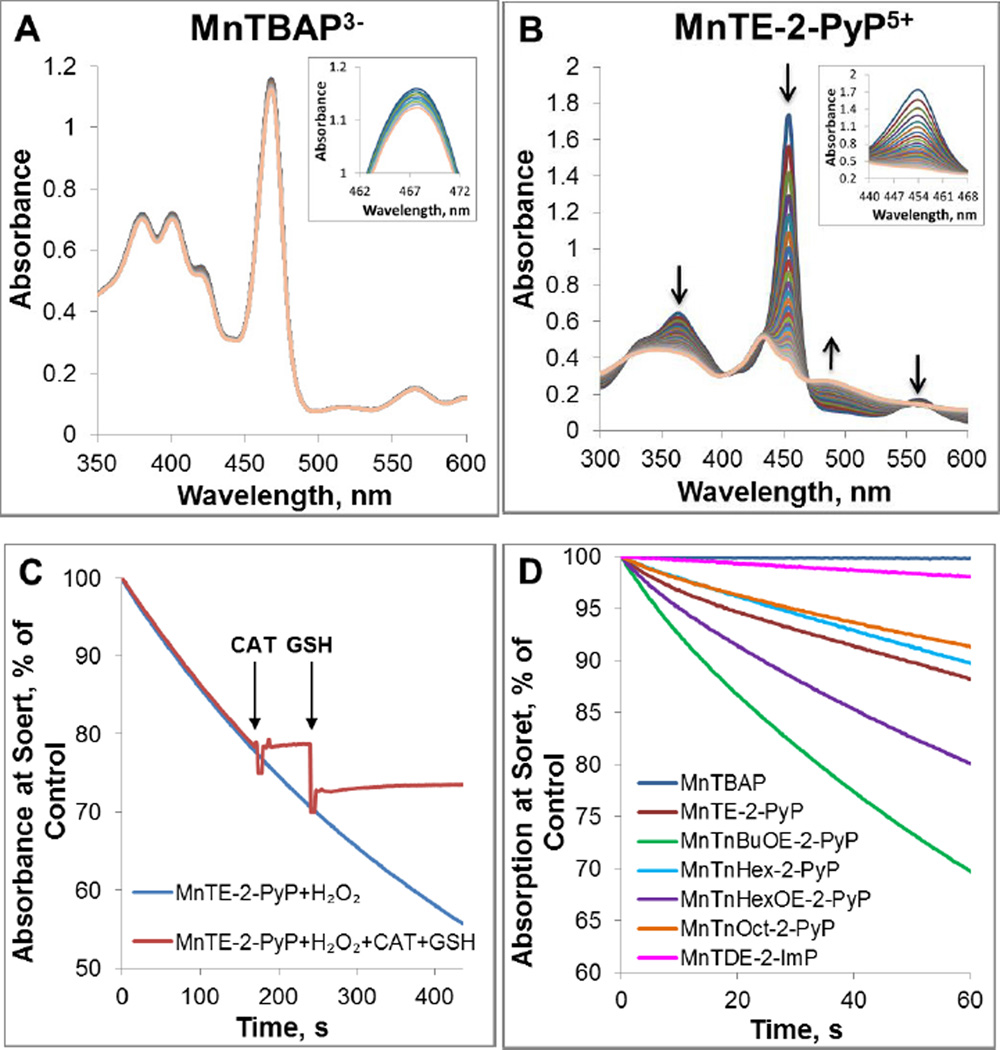
Figure 5. Degradation of Mn porphyrins with H2O2.
Spectral changes were measured within first 30 minutes for Mn porphyrins MnTBAP3− (A) and MnTE-2-PyP5+ (B)). Time-dependent reduction in the absorbance at the Soret band for each MnP is shown in inset. (C) Time-dependent degradation of MnTE-2-PyP5+ with H2O2. The degradation of MnTE-2-PyP5+ was terminated with the addition of catalase. Subsequent addition of GSH did not cause any change in the absorbance indicating irreversible degradation of MnP to non-porphyrin species over the course of ~200 s of reaction. The drop in absorbance is due to a sample dilution. Experiments were carried out at (25±1)°C in 0.05 M tris buffer, pH 7.8 and 0.1 mM EDTA with 10 µM MnP and 0.5 mM H2O2. (D) The H2O2-driven degradation of several MnPs whose structures impose different steric and electronic effects upon H2O2 approach.