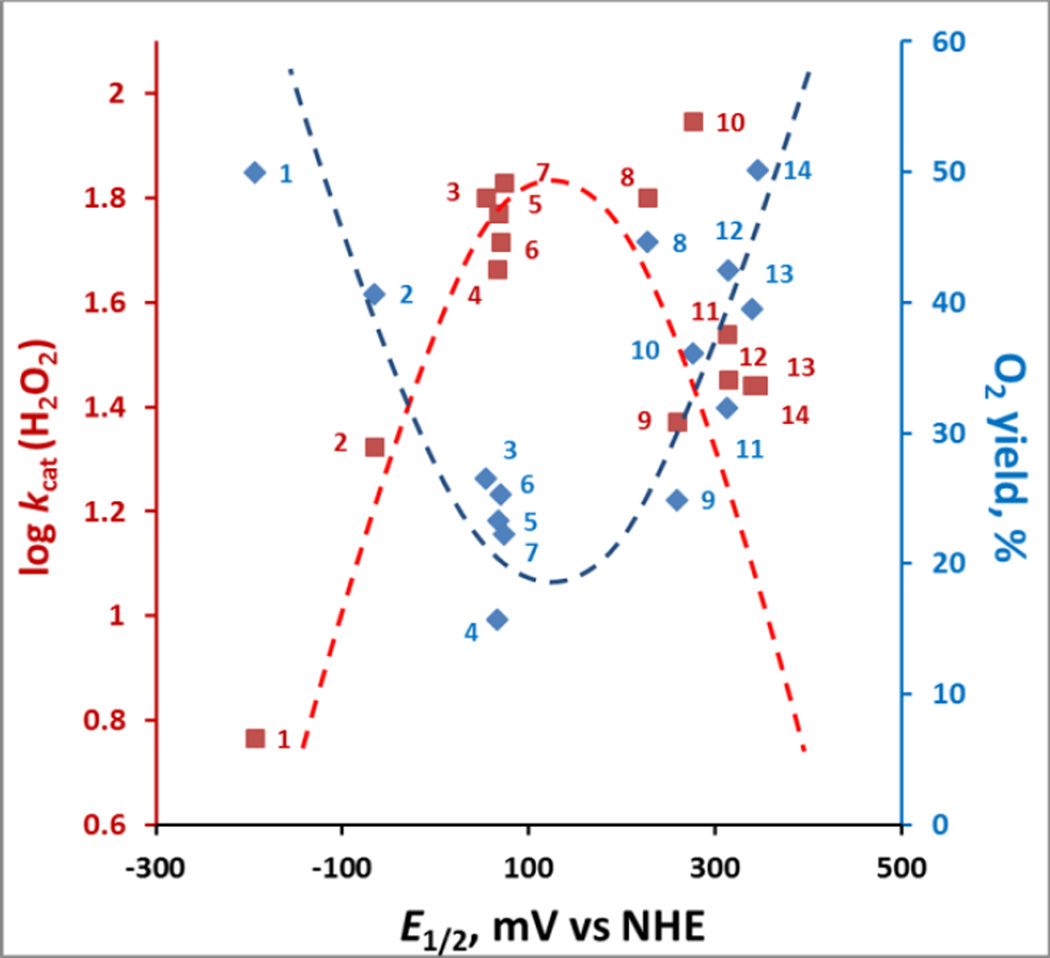
Figure 6. The relationships between the kinetic (log kcat(H2O2)) and thermodynamic parameter (E1/2 for MIII/MII redox couple) each with thermodynamic parameter, yield of O2.
While of similar E1/2, the presence of relatively approachable oxygen atoms in alkoxyalkyl chains in MnTnBuOE-2-PyP5+ (#10) relative to MnTnHexOE-2-PyP5+ (#11) resulted in 2.3-fold higher kcat(H2O2); yet, yields in O2 are identical, due to larger stability of latter compound (Figure 5). MnTDE-2-ImP5+ (#14), with identical kcat(H2O2) but higher stability than MnTnBuOE-2-PyP5+, has higher yield which is also the highest among cationic MnPs. The interplay between the ability of MnPs to catalyze H2O2 dismutation (red line) and their stability to oxidative degradation determines the yield of H2O2 dismutation and the efficacy of catalyst (blue line). Dashed lines are meant to indicate the trends.