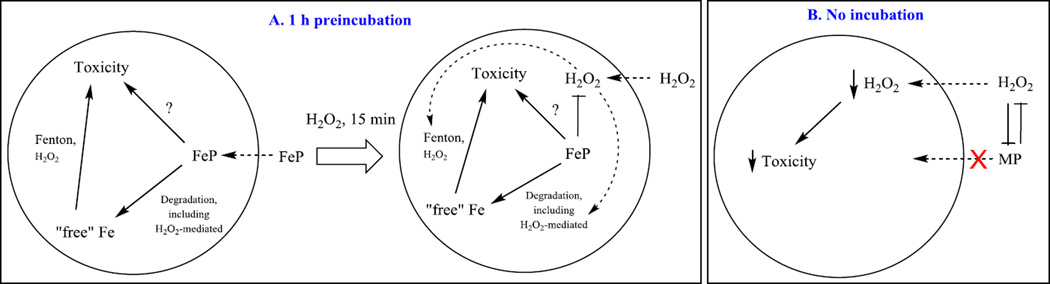
Figure 9. Proposed mechanism of FeP/H2O2 interactions impacting E. coli survival.
In plot (A), FeP was added to E.coli-containing medium for 1 hour prior to 15 min-exposure of culture to H2O2. At 15 min, all H2O2 was removed by the addition of catalase. In plot (B), FeP was added in parallel with H2O2. Under such conditions cycling of FeP with H2O2 releases ”free” Fe from the porphyrin ring, whose uptake is tightly controlled by E.coli; in turn FeP toxicity, seen when cells were preincubated with FeP (Figure 8), was avoided (Figure 7). In pre-incubation scenario (A), the toxicity of FeP may arise from: (i) Fenton chemistry of either “free” Fe released from Fe porphyrin or of Fe site of FeIIP4+; (ii) high-valent oxo FeP species of high oxidizing power; and (iii) direct interaction of FeP with specific cellular proteins/targets. In scenario where FeP catalyst and H2O2 were added simultaneously (plot B), their mutual interaction dismuted (removed) H2O2 only when 0.5 mM H2O2 (LC106) was applied to the cells but not 5 mM (MG1655). Their interaction also degrades FeP and in turn cells do not accumulate it within cell, where, otherwise, it might have caused toxicity as shown in (A). The accumulation of “free” Fe is tightly controlled by cell to avoid Fenton-based toxicity. MP – metalloporphyrin, either MnP or FeP.