Abstract
While cognitive and emotional systems both undergo development during adolescence, few studies have explored top-down inhibitory control brain activity in the context of affective processing, critical to informing adolescent psychopathology. In this study, we used functional magnetic resonance imaging to examine brain response during an Emotional Conflict (EmC) Task across 10–15-year-old youth. During the EmC Task, participants indicated the emotion of facial expressions, while disregarding emotion-congruent and incongruent words printed across the faces. We examined the relationships of age, sex, and gonadal hormones with brain activity on Incongruent vs. Congruent trials. Age was negatively associated with middle frontal gyrus activity, controlling for performance and movement confounds. Sex differences were present in occipital and parietal cortices, and were driven by activation in females, and deactivation in males to Congruent trials. Testosterone was negatively related with frontal and striatal brain response in males, and cerebellar and precuneus response in females. Estradiol was negatively related with fronto-cerebellar, cingulate, and precuneus brain activity in males, and positively related with occipital response in females. To our knowledge, this is the first study reporting the effects of age, sex, and sex steroids during an emotion-cognition task in adolescents. Further research is needed to examine longitudinal development of emotion-cognition interactions and deviations in psychiatric disorders in adolescence.
Keywords: fMRI, Adolescence, Sex Differences, Age, Puberty, Hormones
1. Introduction
Adolescence represents a period of emotional, cognitive, pubertal, psychological, and social maturation. Despite improvements in cognitive and emotional functioning (Durand, Gallay, Seigneuric, Robichon, & Baudouin, 2007; Luna et al., 2001; Tottenham, Hare, & Casey, 2011), this developmental stage has often been characterized as a time of increased vulnerability for the emergence of psychopathology (Dahl & Gunnar, 2009; Ernst & Korelitz, 2009). One prominent theory for this vulnerability is heightened emotional reactivity during adolescence in the face of less mature cognitive control (Dahl, 2004). As a result, neuroimaging studies of adolescent brain development have aimed to understand the neurobiological underpinnings of adolescent emotional and cognitive maturation. Magnetic resonance imaging (MRI) has revealed changes in brain maturation over the course of adolescence, suggesting that brain regions subserving affective and cognitive functions mature at different rates (Mills, Goddings, Clasen, Giedd, & Blakemore, 2014; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999; Sowell, Trauner, Gamst, & Jernigan, 2002). In addition to regionally specific developmental timecourses for subcortical and cortical brain structures (Dennison et al., 2013; Giedd et al., 1999; Gogtay et al., 2004; Mills et al., 2014; Ostby et al., 2009; Sowell et al., 1999; Sowell, Thompson, Tessner, & Toga, 2001; Sowell et al., 2002), it is proposed that the maturation of affective brain regions occurs prior to that of cognitive control regions, resulting in an imbalance between “hot” and “cold” neurocognitive processes during adolescence (Dahl, 2001, 2004; Ladouceur, 2012; Mueller, 2011), and a recent longitudinal study supports this theory (Mills et al., 2014). Thus, the earlier maturational trajectory of subcortical brain regions that subserve bottom-up emotional processing relative to the later trajectory of cortical regions involved in top-down higher order cognitive control may explain some of the vulnerability for the emergence of psychiatric disorders during adolescence.
However, recent reviews present alternative explanations for heightened emotionality and immature cognitive control during adolescence (Crone & Dahl, 2012; Pfeifer & Allen, 2012). These authors point to the mixed evidence for immaturity in the frontal lobe, and suggest that other important factors, such as motivation, social and affective context, training, capacity for learning, flexibility, and task-related factors may better explain changes in behavior during adolescence (Crone & Dahl, 2012; Pfeifer & Allen, 2012). For example, adolescence coincides with a time of increased social and peer engagement, which while evolutionarily adaptive (Steinberg, 2008), has been linked with increased risk-taking and affective disorders, thought to be influenced by pubertal maturation (Forbes & Dahl, 2010; Hamilton, Hamlat, Stange, Abramson, & Alloy, 2014; Smith, Chein, & Steinberg, 2013). Pubertal stage impacts emotional responsivity and associated brain activity (Forbes, Phillips, Silk, Ryan, & Dahl, 2011; Moore et al., 2012), such that limbic and visual cortical processing of emotional faces are associated with puberty at younger ages, while prefrontal cortical processing of affective displays is correlated with puberty at older ages (Moore et al., 2012). While increased prefrontal cortex engagement during emotional face processing may be present in older adolescents, higher levels of testosterone have been shown to reduce the coupling between prefrontal cortex and the amygdala (Spielberg et al., 2014; Volman, Toni, Verhagen, & Roelofs, 2011). These findings emphasize the importance of studying adolescent cognition within emotional contexts.
While emotional processing (e.g. brain activity in response to emotional faces) and cognitive control (e.g. brain activity during response inhibition) are often studied separately in adolescent neuroimaging studies (Adleman et al., 2002; Guyer et al., 2008; Herba, Landau, Russell, Ecker, & Phillips, 2006; Luna et al., 2001; Marsh et al., 2006; Rubia, Smith, Taylor, & Brammer, 2007; Rubia et al., 2006; Tamm, Menon, & Reiss, 2002), top-down executive control and affective processing are often not isolated processes in the environment. Functional magnetic resonance imaging (fMRI) studies of inhibitory control in adolescents show age-related changes in behavior, as well as both greater and less brain activity with age (Luna et al., 2001; Marsh et al., 2006; Rubia et al., 2006; Tamm et al., 2002; Williams, Ponesse, Schachar, Logan, & Tannock, 1999). Developmental changes in behavior and brain response have also been documented in emotional processing studies, many of which suggest increases in emotion recognition abilities across development and relatively higher emotion-related brain activity during adolescence compared with other periods of life (Durand et al., 2007; Gao & Maurer, 2010; Guyer et al., 2008; Herba et al., 2006; Pfeifer et al., 2011). However, few studies of emotion-cognition interactions during development are present in the adolescent literature. Existing studies have examined emotional processing effects on inhibitory control during go-nogo tasks (Hare et al., 2008; Schel & Crone, 2013; Somerville, Hare, & Casey, 2011; Tottenham et al., 2011), cognitive interference during an emotional Stroop task (Mincic, 2010), and the effects of emotional valence on interference during a working memory task (Ladouceur et al., 2005). With respect to emotion-cognition interactions, only one of these studies used fMRI to examine developmental effects and found a negative association between age and inferior frontal gyrus activity during response inhibition (Somerville et al., 2011). Due to limited knowledge of how these different neural networks interact during adolescence, further research on emotion-cognition interactions is needed to improve our understanding of the maturational changes occurring simultaneously within these systems. By understanding how these systems are engaged during typical adolescent brain development, researchers will be better able to interpret brain activity observed in internalizing and externalizing disorders that emerge during adolescence.
Another important factor to consider during development is sex differences, which exist both structurally (see Giedd, Raznahan, Mills, and Lenroot (2012) for review) and functionally (Rubia, Hyde, Halari, Giampietro, & Smith, 2010; Rubia et al., 2013; Schneider et al., 2011; Schweinsburg, Nagel, & Tapert, 2005) in the adolescent brain. Functional differences between the sexes have been observed during attention allocation (Rubia et al., 2010), inhibitory control (Christakou et al., 2009; Rubia et al., 2013), emotional processing in the amygdala (Schneider et al., 2011), and spatial working memory (Alarcon, Cservenka, Fair, & Nagel, 2014; Schweinsburg et al., 2005) in adolescents. Specifically, during inhibitory control, left hemispheric fronto-striatal activity is greater in girls, while parietal activity is greater in boys, and age-by-sex interactions in these same regions are believed to reflect sex-specific changes in functional maturation (Christakou et al., 2009; Rubia et al., 2013). Not only are brain volume and activity differences present between the sexes, but the prevalence of psychiatric disorders also varies between males and females, such that externalizing disorders are more often seen in males, while internalizing disorders are more prevalent in females (Earls, 1987). Understanding sex differences in healthy adolescent brain response during emotion-cognition interactions may help explain sex-specific rates of occurrence of these disorders.
In addition to age-related and sex-specific changes in brain maturation, pubertal status has also been shown to impact brain structure (Blanton et al., 2012; Giedd et al., 2006; Goddings et al., 2014) and function (Forbes et al., 2011; Goddings, Burnett Heyes, Bird, Viner, & Blakemore, 2012; Klapwijk et al., 2013; Moore et al., 2012). Functional studies suggest that stage of pubertal development is associated with changes in socio-affective response and connectivity during adolescence (Forbes et al., 2011; Goddings et al., 2012; Klapwijk et al., 2013; Moore et al., 2012). For example, pre-/early pubertal adolescents show relatively heightened amygdalar and ventrolateral prefrontal cortex activity to emotional faces, compared with mid/late-pubertal adolescents (Forbes et al., 2011). During this time, they also show increases in amygdalar, hippocampal, and temporal lobe activity, and activation in prefrontal areas, previously unobserved in childhood (Moore et al., 2012). Furthermore, these studies suggest that underlying hormonal changes may, in part, explain why pubertal status accounts for some of the variance in brain development. Structural MRI (Bramen et al., 2012; Bramen et al., 2011; Herting et al., 2014; Peper et al., 2009), diffusion tensor imaging (Herting, Maxwell, Irvine, & Nagel, 2012), and fMRI (Goddings et al., 2012; Klapwijk et al., 2013; Op de Macks et al., 2011) studies have found relationships between sex steroids and brain anatomy and activity, respectively, in healthy adolescent samples. Pubertal maturation may be particularly relevant to the development of brain systems underlying social and reward-related processing during adolescence. For example, testosterone and estradiol are positively associated with social vs. basic emotional processing brain activity (Goddings et al., 2012), with estradiol relating to greater connectivity among social brain processing regions in girls (Klapwijk et al., 2013). These findings could underlie adolescent movement towards emotionally salient peer relationships. Further, reward-related striatal response is positively associated with testosterone in both males and females (Op de Macks et al., 2011), further supporting hormonal roles in affectively and/or motivationally driven neural systems. Thus, studies examining hormone levels and brain activity in healthy adolescents may help inform future studies of atypical neurodevelopment, where hormone levels or their influence on brain structure and/or functioning may be altered.
To expand upon existing literature and examine the impact of the aforementioned variables, we implemented a modified Emotional Conflict (EmC) Task (Etkin, Egner, Peraza, Kandel, & Hirsch, 2006) in a typically developing adolescent population. During the EmC Task, participants were required to indicate the emotion of facial expressions while disregarding emotion-congruent and incongruent words printed across the faces, with conflict being defined as brain activity differences between Incongruent vs. Congruent trials. Therefore, in this task participants must dedicate attentional resources during the Incongruent trials to the target stimulus, while overcoming distraction from the conflicting emotion printed across the face. These attentional demands depend partly on the engagement of dorsal anterior cingulate cortex and dorsomedial cortex activity (Etkin et al., 2006). The main goals of this study were to examine a) age-related effects on emotional conflict-related behavior and brain activity, and b) sex differences in behavior and brain activity, controlling for pubertal status. Our behavioral hypotheses were that age would predict improvements in accuracy and reaction time on the task, and that these would be most pronounced during emotional conflict trials. Based on the overall lack of sex differences (except for Rubia et al. (2010) and Tottenham et al. (2011)) examined or reported in the majority of previous behavioral and fMRI studies of inhibitory control, we hypothesized that behavioral differences between males and females would be non-significant. At the neural level, we predicted that age would positively relate to the engagement of top-down cognitive control and attentional processing regions, such as dorsal anterior cingulate, dorsomedial, and dorsolateral prefrontal cortices, during emotional conflict. Further, based on the limited number of previous studies examining sex differences in brain response during response inhibition and cognitive interference across development (Christakou et al., 2009; Rubia et al., 2013), we predicted that sex differences in brain activity would be present in fronto-striatal and parietal regions (Christakou et al., 2009; Rubia et al., 2013), after controlling for pubertal development. Finally, we conducted an exploratory analysis to examine the effect of both estradiol and testosterone on emotional conflict-related brain activity.
2. Material and Methods
2.1 Participant Characteristics
Participants consisted of a community sample of 44 healthy children and adolescents ages 10 to 15 (mean age = 13.28 ± 1.56; median age = 13.12, 22 female). Following informed consent and assent in accordance with the Oregon Health & Science University (OHSU) Institutional Review Board, all participants and a parent/guardian underwent separate comprehensive screening interviews to assess the youth’s eligibility. Exclusionary criteria for youth included a current or personal history of DSM-IV psychiatric disorders [Diagnostic Interview Schedule for Children Predictive Scales, (Lucas et al., 2001)], major medical conditions that require pharmacological management and/or those affecting the central nervous system, head injury (loss of consciousness >2 minutes), mental retardation or learning disability, premature birth, prenatal exposure to alcohol/drugs, significant personal alcohol/drug use ( >10 lifetime alcoholic drinks or >2 drinks per occasion, >5 uses of marijuana, any other drug use, or >4 cigarettes per day) [Customary Drug Use and Drinking Record, (Brown et al., 1998)], a history of psychotic illness in a biological parent, left-handedness [Edinburgh Handedness Inventory, (Oldfield, 1971)], pregnancy, use of contraceptive medication, or other MRI-incompatibility.
Participants were administered the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) to estimate overall intellectual functioning. Puberty was self-reported using Pubertal Development Scale Crockett Staging (Petersen, Crockett, Richards, & Boxer, 1988), which classifies pubertal development on a scale ranging from stage 1 (pre-puberty) through stage 5 (post-puberty) (Carskadon & Acebo, 1993), and is based on an unpublished manuscript (Crockett, 1988). The Hollingshead Index of Social Position was used to estimate socioeconomic status (SES) based on the occupation and education level of each parent (Hollingshead, 1957).
2.2 Hormonal Assessment
Within one week of image acquisition, 4 milliliters of blood was collected from participants via venapuncture between the hours of 7:00 and 10:00AM. Blood draws for post-menarche females occurred during the first 10 days of their menstrual cycle (follicular phase) to reduce variability associated with menstrual phase. Total testosterone levels were determined by Coat-A-Count radioimmunoassay (Diagnostic Product Corp., Los Angeles, CA). The intra-assay coefficient of variation (CV) was 7.0% and the inter-assay CV was 8.8%. The lower level limit for detection was 10 ng/dL. Data from 3 males and 9 females were excluded due to testosterone levels ≤ 10 ng/dL, the limit for reliable detection. Thus, the final sample size for testosterone analyses included 19 males and 13 females. Estradiol levels were determined using the DSL-4800 Ultra-sensitive Estradiol Radioimmunoassay Kit (Beckman Coulter, Fullerton, CA). The intra-assay and inter-assay CVs were 6.9% and 9.0%, respectively, with a lower level limit for detection of 2.2 pg/mL. Estradiol data were missing for three female participants, so the final sample for this analysis included 22 males and 19 females. No youth had values under the detection limit for estradiol.
2.3 Emotional Conflict Task
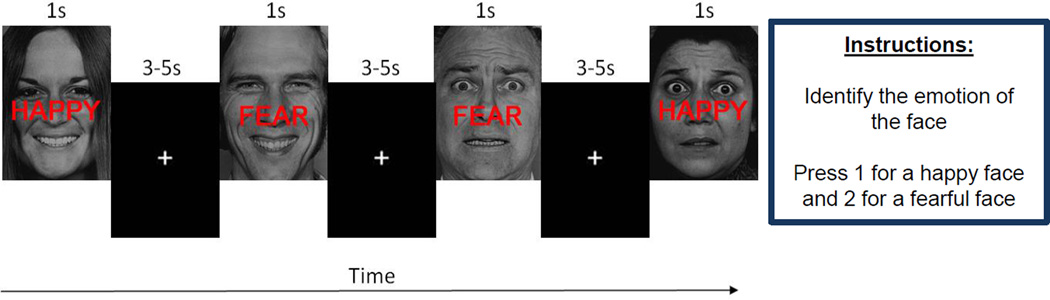
To assess the neural substrates of emotional conflict, participants performed a previously published version (Etkin et al., 2006) of the EmC Task (Figure 1) programmed in Presentation software (Neurobehavioral Systems, http://nbs.neuro-bs.com). This emotional processing task required participants to identify the expression of an emotional face, while disregarding emotion-congruent or incongruent words printed across the face. The task included six different female and five different male actors selected from the Pictures of Facial Affect dataset (Ekman & Friesen, 1976). Half of the trials were emotion-congruent, such that the word “happy” or “fear” was superimposed on a face with a corresponding emotional expression. The other half were emotion-incongruent, where the word “happy” or “fear” was superimposed on a face with a contrasting emotional expression. Participants were instructed to indicate the emotional expression of the face with a button press and disregard the word. They were also instructed to respond as quickly and accurately as possible. Data were collected during a single functional run lasting 13:14 minutes, which included 148 trials. These trials were divided into 74 emotion-incongruent (38 fearful/36 happy), and 74 emotion-congruent (36 fearful/38 happy) expressions. The 148 trials included 76 male faces from the five different male actors, and 72 female faces from the six different female actors. Trials were 1 second long with intertrial fixation intervals jittered between 3–5 seconds. Prior to scanning, participants completed a minimum of 23 practice trials to ensure understanding of the task.
Figure 1. Emotional Conflict Task.
Example stimuli used in the Emotional Conflict Task. Participants were asked to identify the emotional expression of a face while disregarding the emotion-congruent or incongruent word printed across the face. For half of the trials, the word “happy” or “fear” was superimposed on a face with the corresponding emotional expression (Congruent trials). For the other half, the word “happy” or “fear” was superimposed on a face with the contrasting emotional expression (Incongruent trials). Trials were 1 second long with intertrial fixation intervals jittered between 3–5 seconds.
2.4 Imaging
2.4.1 Image Acquisition
Participants were scanned at OHSU’s Advanced Imaging Research Center on a 3.0 Tesla Siemens Magnetom Tim Trio with a 12 channel head coil. Prior to administration of the EmC Task, a whole-brain high-resolution T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) structural sequence was acquired in the sagittal plane (time repetition (TR) = 2300 ms, time to echo (TE) = 3.58 ms, inversion time (TI) = 900 ms, flip angle = 10°, field of view (FOV) = 256 × 240 mm, voxel size = 1 × 1 × 1.1 mm, scan time = 9:14). A functional T2*-weighted echo planar imaging sequence was acquired in the axial plane parallel to the anterior commissure – posterior commissure line (TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 240 mm2, voxel size = 3.75 × 3.75 × 3.8 mm, 33 slices, scan time = 13:14).
2.4.2 Image Preprocessing
FMRI preprocessing followed previously published procedures (Cservenka & Nagel, 2012). Briefly, following image reconstruction, slice timing correction, spatial normalization to the frame requiring the least amount of spatial adjustment (Cox & Jesmanowicz, 1999), and co-registration of structural and functional data were performed. Next, a 6 mm full-width half maximum Gaussian kernel was applied to the functional data for smoothing to increase signal-to-noise ratio (SNR). Signal normalization was then applied to later extract values as relative percent signal change in the blood oxygen level-dependent (BOLD) response. To further limit the impact of head movement on the SNR, TRs requiring greater than 2.5 mm or 2.5 degrees translation or rotation, respectively, during spatial normalization, were censored prior to further image processing. AFNI’s 3dREMLFit was used to model the hemodynamic response function, while accounting for delays in the response, using a boxcar function (Cox, 1996). Regressors of interest included Happy Congruent and Incongruent trials, as well as Fear Congruent and Incongruent trials, while incorrect trials and the 3 rotational and 3 translational movement parameters were included as nuisance regressors. Contrasts for this model compared brain activity during Incongruent vs. Congruent correct trials. Each participant’s activation map was transformed to 3 mm3 voxels in Talairach atlas space (Talairach & Tournoux, 1988). To compare head movement between males and females, and in relation to age, root mean square (RMS) was calculated across the entire run for each participant. There was a significant association between age and RMS, such that older participants had lower RMS values, indicative of less head movement (F(1,42) = 4.08, β = −0.30, t = −2.02, p = 0.0498). There were no significant differences in head movement between males (M = 0.34, SD = 0.27) and females (M = 0.27, SD = 0.17), (t = −1.09, p = 0.28).
2.5 Group Analyses
2.5.1 Demographic and Behavioral Data
Statistical analyses were performed in IBM SPSS Version 20.0 (Corp., Released 2011). Demographic data were analyzed using independent-samples t-tests, and the appropriate nonparametric tests when data violated the assumption for normality (see Table 1 for participant characteristics). In addition, as this study was interested in examining developmental effects on emotional conflict-related behavior and brain activity, correlations of task accuracy and reaction time were performed with the four task conditions (Happy-Congruent, Fear-Congruent, Happy-Incongruent, Fear-Incongruent) to examine whether accuracy or reaction time on these measures differed between the positive and negative emotional faces, with respect to age, and whether the two emotional conditions resulted in significantly different behavioral performance between the sexes. The confidence intervals for the age-related correlations were highly overlapping (see Table 2), and there were no significant performance differences between the two emotional conditions in either males or females (Table 3). As a result, Happy and Fearful Congruent trials were combined to represent “Congruent” brain response, while Happy and Fearful Incongruent trials were combined to represent “Incongruent” brain activity in the imaging analyses that follow.
Table 1. Participant characteristics.
Values represent mean (SD), unless otherwise noted. The groups were significantly different on pubertal and hormonal measures but not on any of the other measures.
| Males (n=22) | Females (n=22)f | Statistic | |
|---|---|---|---|
| Age | 13.47 (1.51) | 13.10 (1.62) | t42 = 0.80 |
| Pubertal Statusa | 3.00 (2.00) | 4.00 (1.00) | U42= 157.5, Z = −2.07* |
| IQb | 117.14 (11.14) | 113.45 (10.95) | t42 = 1.11 |
| SESc | 27.09 (10.02) | 25.00 (11.12) | U42= 219.5, Z = −.53 |
| Caucasian (%) | 90.90 | 90.90 | |
| Testosteroned (ng/dL) Range |
320.3 (175.14) 27.5 – 639.0 |
22.23 (12.64) 10.1 – 47.4 |
U30= 4.0, Z = −4.59* |
| Estradiole (pg/mL) | 16.27 (7.45) | 23.67 (13.0) | t39 = 2.23* |
| Range | 4.97 – 34.10 | 7.7 – 53.8 |
Pubertal Developmental Scale Crockett Stage; scores range 1–5, with higher scores reflecting greater maturity (Petersen et al., 1988); values represent median (interquartile range). The PDS has equivalent questions for growth in height, body hair, and skin changes for boys and girls, while questions regarding breast development, menstruation, voice changes, and facial hair are sex-specific.
Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)
Hollingshead Index of Social Position; higher scores indicate lower socioeconomic status (Hollingshead, 1957)
N=19 males and 13 females due to values below the detection limit (< 10 ng/dL) that were excluded from subsequent analyses
N=22 males and 19 females due lack of blood collected to be sufficient for both hormone assays for those three girls.
Nine of the 22 girls were premenstrual. Of the 13 postmenstrual girls, three reported irregular cycles, but these girls were also brought into the study during the first 10 days following menstruation.
p < 0.05
Table 2. Age correlations.
Pearson correlation coefficients are presented in each column, with 95% confidence interval values below.
| Happy | Fear | |||
|---|---|---|---|---|
| Congruent Accuracy (%) | ||||
| r | .325 | .110 | ||
| CI | .031 | .620 | −.199 | .420 |
| Congruent RT (ms) | ||||
| r | −.289 | −.304 | ||
| CI | −.587 | .009 | −.601 | −.007 |
| Incongruent Accuracy (%) | ||||
| r | .439 | .423 | ||
| CI | .159 | .719 | .141 | .705 |
| Incongruent RT (ms) | ||||
| r | −.304 | −.309 | ||
| CI | −.601 | −.008 | −.605 | −.012 |
Table 3. Differences in behavioral performance by emotion in males and females.
Results of paired samples t-tests comparing performance (accuracy and reaction time) on happy and fearful trials in males and females. There were no significant differences on any of the performance measures in males or females.
| Males | Females | |
|---|---|---|
| Congruent Accuracy (Happy vs. Fear) | t21 = 1.58; p =.13 | t21 = 1.63; p =.12 |
| Congruent RT (Happy vs. Fear) | t21 = −1.01; p =.32 | t21 = −1.49; p =.15 |
| Incongruent Accuracy (Happy vs. Fear) | t21 = 0.46; p =.65 | t21 = 1.45; p =.16 |
| Incongruent RT (Happy vs. Fear) | t21 = 0.20; p =.84 | t21 = −.82; p =.42 |
2.5.2 Imaging Data
To examine age-related effects on emotional conflict-related brain activation, a regression model was built using AFNI’s 3dttest++ with age, sex (dummy-coded), and age-by-sex interactions included. This allowed examination of age-related associations with Incongruent vs. Congruent brain response (emotional conflict), independent of sex, as well as age-by-sex interactions. Specifically, we examined the effect of each predictor, while controlling for the other variables in the model. First, youth were split into “young” and “old” age groups using a median split, and individual one-sample t-tests were used to generate voxel thresholded (p < 0.05) maps of each group. These were then added together to form a task-related activity map. The regression model was restricted to the task-related activity map to best capture brain activity that was significantly associated with the task contrast (Incongruent vs. Congruent). A separate model was used to examine sex differences in brain activity, while controlling for pubertal stage, and testing sex-by-puberty interactions. Similarly, for this model, male and female task-related activity maps (p < 0.05) were generated with one-sample t-tests and added together to form a task-related activity map, thereby capturing any distinct sex-related patterns of brain response in a restricted map to which the regression model was then applied. Although scores from the PDS are categorical, this measure was included in parametric analyses, to conduct all brain level analyses using general linear models. Finally, due to the large variability in sex steroid values between males and females, additional regression models examined the specific contributions of testosterone and estradiol to emotional conflict-related brain response in males and in females separately. Task-related masks for these models were created by using a median split of hormone values for each of the four analyses, creating voxel thresholded task-related maps (p < 0.05) for “low” and “high” hormone levels separately, adding the masks, and examining the effects of the hormones within these task-related masks. The hormone models controlled for age post-hoc to capture all variance associated with hormones, but verify that hormonal relationships drove the results in significant clusters, above and beyond variance accounted for by age.
Three separate models were chosen for the analyses, as to avoid the presence of multicollinearity among highly correlated variables, and to preserve degrees of freedom. All main effects and interactions were corrected for multiple comparisons by using Monte Carlo simulation with a voxel (p < 0.01) and cluster threshold (α < 0.05). The minimum cluster sizes varied for each model, depending on the task-related mask used: age-related model: 15 voxels, sex differences model: 20 voxels, testosterone in boys: 13 voxels, testosterone in girls: 19 voxels, estradiol in boys: 16 voxels, and estradiol in girls: 16 voxels. For the first two models, significant clusters were extracted, and post-hoc hierarchical regressions were conducted in SPSS to control for accurate performance (z-score of Congruent accuracy and reaction time and z-score of Incongruent accuracy and reaction time), as well as movement (RMS) in these clusters. Age was controlled for with post-hoc hierarchical regressions for the hormone analyses to determine whether hormones predicted brain activity in the significant clusters, above and beyond age. Significant clusters from the first two model models were surface mapped onto the Population-Average, Landmark, and Surface-based (PALS-B12) atlas (Van Essen et al., 2001), while results from the third model are displayed in volume space, using AFNI’s anatomical template as the underlay, to better illustrate subcortical and cerebellar findings.
3. Results
3.1 Demographic and Behavioral Characteristics
Males and females were matched on all demographic characteristics, except for pubertal stage, which was significantly greater in females, and sex steroid levels, which were significantly higher for testosterone in males and for estradiol in females (Table 1). There were no performance differences on accuracy or reaction time on either Congruent or Incongruent trials between males and females (≥ 70 % accuracy on each condition for all youth). However, age correlations showed significant negative relationships with Congruent and Incongruent reaction time across the participant sample, as well as a significant positive correlation between age and Incongruent trial accuracy, as well as pubertal stage and Incongruent trial accuracy. There was also a trend-level positive association between Congruent trial accuracy and age (Table 4). There were no significant sex differences in the contrasts of Incongruent vs. Congruent accuracy or reaction time. However, the Incongruent vs. Congruent accuracy contrast was significantly related to age and pubertal stage (Table 4). In males, estradiol was significantly negatively related to reaction time on the task, while in females, estradiol was significantly positively related to Congruent trial accuracy. No significant associations with testosterone and task performance were found for either males or females (Table 4).
Table 4. Behavioral performance.
Means are presented in each column, with standard deviations for each group presented in parentheses.
| Males | Females | Statistic | Age Correlation (n=44) |
Pubertal Stage Correlation (n=44) |
Testoster one (Males n=19) |
Testoster one (Females n=13) |
Estrodial (Males n=22) |
Estrodial (Female n=19) |
|
|---|---|---|---|---|---|---|---|---|---|
| Congruent ACC (%) | 94.10(4.96) | 94.90(3.91) | t42 =.59 | r = .25^ | ρ = .11 | r = −.36 | r = .18 | r = .20 | r = .50* |
| Congruent RT (ms) | 758.99(168.18) | 824.63(163.52) | t42 =1.31 | r = −.30* | ρ = −.23 | r = −.20 | r = −.47 | r = −.45* | r = 42^ |
| Incongruent ACC(%) | 87.41(8.16) | 88.76(7.88) | t42 =.56 | r = .48*** | ρ = .40* | r = −.29 | r = .19 | r = .36 | r = −.08 |
| Incongruent RT(ms) | 817.39(178.63) | 885.69(196.72) | t42 =1.21 | r = −.31* | ρ = −.30^ | r = −.22 | r = −.40 | r = −.49* | r = −.30 |
| Incongruent -Congruent ACC (%) | −6.70(4.54) | −6.14(6.80) | t42 =.32 | r = .47*** | ρ =.43* | r = −.07 | r = .14 | r =42^ | r= −.36 |
| Incongruent −Congruent RT (ms) | 58.40(39.69) | 61.06(50.02) | t42 =.20 | r = −.17 | ρ = .04 | r = −.14 | r = −.06 | r = −.30 | r = .19 |
Males and females were not significantly different on any of the behavioral measures presented;
p ≤ .1,
p ≤ .05,
p ≤ .001.
ACC = accuracy
RT = reaction time
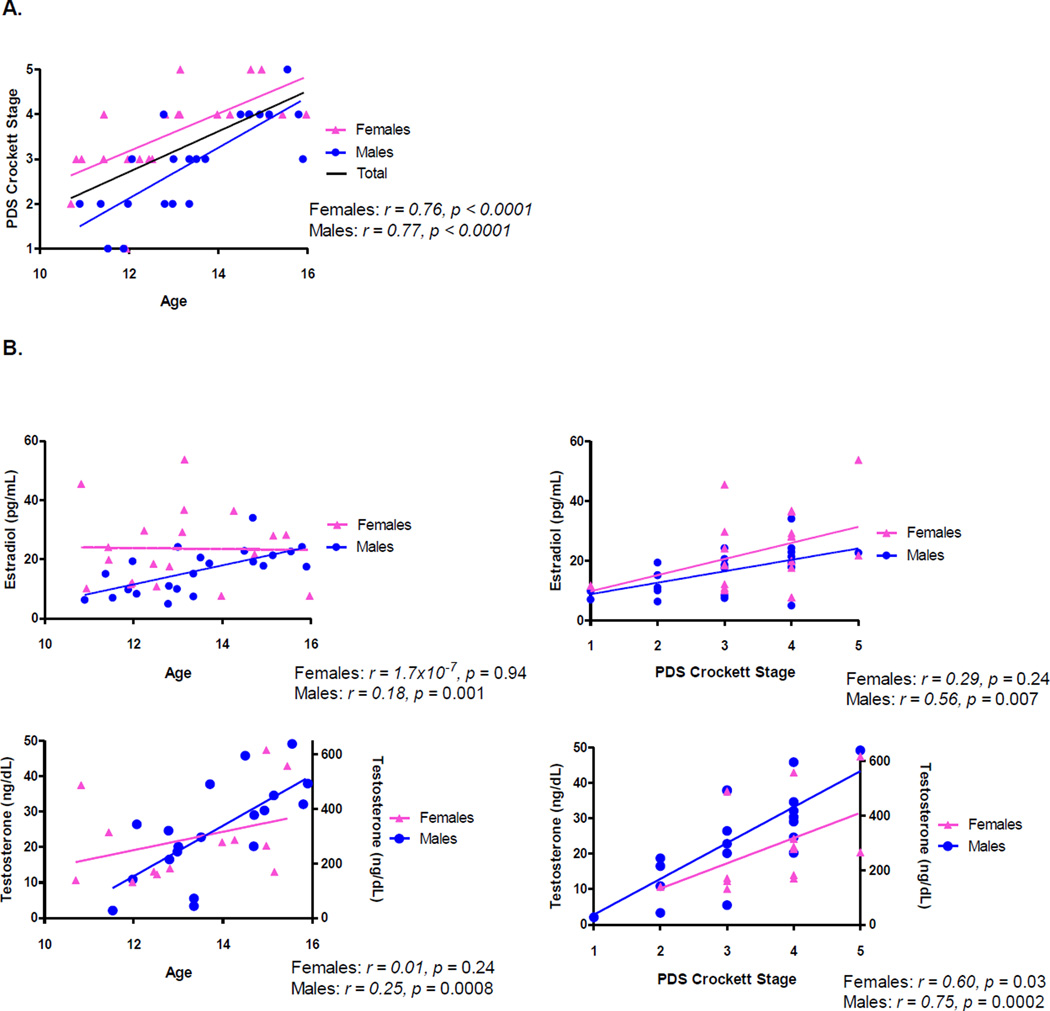
Age and pubertal stage were significantly related in both males and females. Both testosterone and estradiol were significantly related to age and pubertal stage in males, while only testosterone and pubertal stage were significantly related in females (Figure 2).
Figure 2. Age, pubertal stage, and hormone relationships by sex.
a) Significant correlation between age and PDS in females and males, b) significant correlations between age and hormones, as well as PDS and hormones in males, with only PDS and testosterone being significantly related in females. Pearson’s r values for age and hormone correlations, and Spearman’s r values for all correlations with PDS. Only youth with hormone values above the detection limit are included in the correlations. Graphs with testosterone have values on the left y-axis for girls, and right y-axis for boys.
3.2 Model 1: Age-Related Relationships with Brain Activity
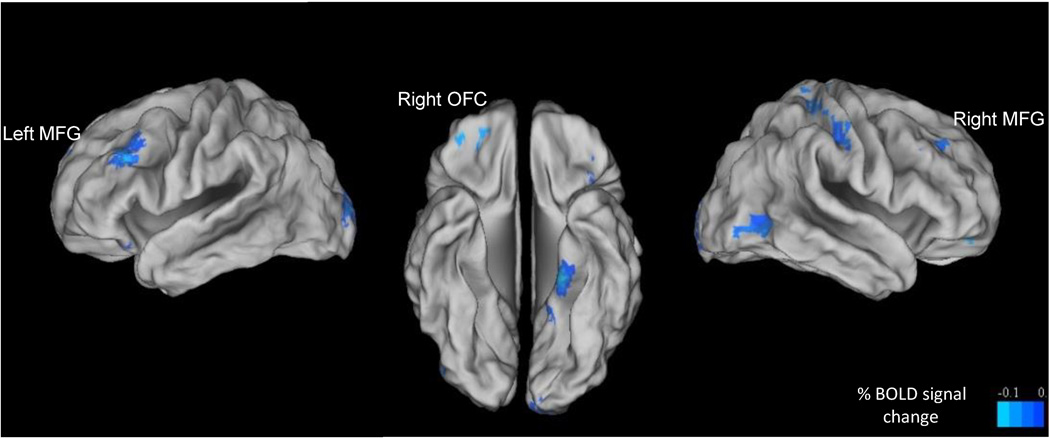
In the first model, two significant clusters of Incongruent vs. Congruent brain activity were found to be related to age, after controlling for task performance and movement. These clusters were located in the left and right middle frontal gyrus (MFG). There was also a trend for a significant association between age and brain activity in the right orbitofrontal cortex (OFC; x = 35, y = 47, z = −10) (Figure 3/Table 5). In all of these brain regions, there was a negative relationship between age and emotional conflict-related BOLD response (Table 6). There was no significant age-by-sex interaction in this model. Brain activity between Happy Congruent and Fear Congruent trials, as well as Happy Incongruent and Fear Incongruent trials was compared to confirm that results were not driven by a specific emotional face valence. Paired samples t-tests indicated no significant differences in the reported clusters (all p’s > 0.10).
Figure 3. The effects of age on emotional conflict-related brain response.
Brain activity was significantly negatively related to age, while controlling for sex and age by sex interactions in bilateral middle frontal gyrus, while a trend-level association was present in the orbitofrontal cortex (voxel/clusterwise corrected, p/α < 0.01/0.05, minimum cluster size: 15 voxels). These associations were present after using post-hoc hierarchical regressions to control for task performance and head movement. Blood oxygen level-dependent activity maps are surface mapped onto the Population-Average, Landmark-, and Surface-based (PALS-B12) atlas in Talairach space (Van Essen et al., 2001). MFG = middle frontal gyrus; OFC = orbitofrontal cortex.
Table 5.
Significant age, sex, and hormone-related effects on emotional conflict-related brain response.
| Peak Talairach | ||||||
|---|---|---|---|---|---|---|
| Main Effect | N | Peak Anatomical Location |
Number of Voxels |
x | y | z |
| Age | 44 | Negative relationship |
||||
| R MFG | 73 | 44 | 14 | 51 | ||
| L MFG | 30 | −50 | 26 | 36 | ||
| Sex | 22M/22F | Boys > Girls | ||||
| R Cuneus | 85 | 8 | −68 | 33 | ||
| R Precuneus | 62 | 2 | −47 | 57 | ||
| R IPL | 53 | 50 | −35 | 30 | ||
| L Fusiform Gyrus | 34 | −29 | −68 | −10 | ||
| R IFG | 26 | 47 | 23 | 9 | ||
| R MOG | 24 | 32 | −80 | 15 | ||
| Testosterone (Boys) |
19 | Negative relationship |
||||
| L MFG | 26 | −29 | 5 | 60 | ||
| L Putament/lentiform nucleus |
21 | −29 | −17 | 6 | ||
| Testosterone (Girls) |
13 | Negative relationship |
||||
| R Cerebellar tonsil | 42 | 23 | −32 | −37 | ||
| L Cerebellar tonsil | 30 | −23 | −38 | −37 | ||
| L Precuneus | 20 | −5 | −80 | 39 | ||
| Estradiol (Boys) |
22 | Negative relationship |
||||
| L Tuber (cerebellum) |
92 | −38 | −65 | −25 | ||
| L SFG | 68 | −2 | 20 | 63 | ||
| L Culmen (cerebellum) |
48 | −14 | −26 | −13 | ||
| L IFG | 36 | −41 | 26 | −19 | ||
| R Cingulate Gyrus | 24 | 5 | −5 | 21 | ||
| R Precuneus | 18 | 23 | −59 | 42 | ||
| L MFG | 17 | −38 | 5 | 57 | ||
| R Pyramis (cerebellum) |
16 | 29 | −68 | −28 | ||
| Estradiol (Girls) |
19 | R IOG | 16 | 29 | −95 | −4 |
IFG = inferior frontal gyrus
IOG = inferior occipital gyrus
IPL = inferior parietal lobule
MFG = middle frontal gyrus
MOG = middle occipital gyrus
L = left
R = right
SFG = superior frontal gyrus
Table 6.
Full model statistics for age, sex, and hormone-related effects on emotional conflict-related brain response, controlling for nuisance variables.
| Statistics for Age and Sex Effects | ||||||||
|---|---|---|---|---|---|---|---|---|
| Main Effect |
Peak Anatomical Location |
F | R2 | p | β | t | p | f2f |
| Agea | Negative relationship |
|||||||
| R MFG | (4,39)=3.18 | 0.25 | 0.02 | −0.38 | −2.25 | 0.03 | 0.13 | |
| L MFG | (4,39)=3.08 | 0.24 | 0.03 | −0.40 | −2.32 | 0.03 | 0.14 | |
| R OFC | (4,39)=2.46 | 0.20 | 0.06 | −0.41 | −2.31 | 0.03 | 0.14 | |
| Sexb | Boys > Girls | |||||||
| R Cuneus | (4,39)=3.38 | 0.26 | 0.02 | 0.52 | 3.67 | 0.001 | 0.35 | |
| R Precuneus | (4,39)=3.99 | 0.29 | 0.008 | 0.49 | 3.55 | 0.001 | 0.32 | |
| R IPL | (4,39)=3.69 | 0.27 | 0.01 | 0.53 | 3.77 | 0.001 | 0.36 | |
| L Fusiform Gyrus |
(4,39)=3.31 | 0.25 | 0.02 | 0.41 | 2.90 | 0.006 | 0.22 | |
| R IFG | (4,39)=5.90 | 0.38 | 0.001 | 0.57 | 4.41 | <0.001 | 0.50 | |
| R MOG | (4,39)=6.42 | 0.40 | <0.001 | 0.57 | 4.44 | <0.001 | 0.51 | |
| Statistics for Hormone Relationships | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | R2g | p | β(age) | t | p | β(H) | t | p | f2 | ||
| Testostero nec (Boys) |
Negative relationship |
||||||||||
| L Putamen/lentiform nucleus |
(2,16)=8.78 | 0.52 | 0.003 | −0.16 | −0.68 | 0.51 | −0.60 | −2.46 | 0.03 | 0.38 | |
| L MFG | (2,16)=8.42 | 0.51 | 0.003 | 0.03 | 0.11 | 0.91 | −0.74 | −2.99 | 0.009 | 0.56 | |
| Testosteroned (Girls) | Negative relationship |
||||||||||
| R Cerebellar tonsil |
(1,11)=19.11 | 0.64 | 0.001 | −0.80 | −4.37 | 0.001 | |||||
| L Cerebellar tonsil |
(1,11)=10.24 | 0.48 | 0.008 | −0.69 | −3.20 | 0.008 | |||||
| L Precuneus | (1,11)=9.88 | 0.47 | 0.009 | −0.69 | −3.14 | 0.009 | |||||
| Estradiol (Boys)e |
Negative relationship |
||||||||||
| R Tuber (cerebellum) |
(2,19)=9.65 | 0.50 | 0.001 | −0.32 | −1.49 | 0.15 | −0.46 | −2.17 | 0.043 | 0.25 | |
| L SFG | (2,19)=17.37 | 0.65 | <0.001 | −0.51 | −2.81 | 0.01 | −0.38 | −2.11 | 0.05 | 0.23 | |
| L Culmen (cerebellum) |
(2,19)=11.33 | 0.54 | 0.001 | −0.35 | −1.73 | 0.10 | −0.46 | −2.24 | 0.04 | 0.27 | |
| L IFG | (2,19)=17.91 | 0.65 | <0.001 | −0.47 | −2.64 | 0.02 | −0.42 | −2.36 | 0.03 | 0.29 | |
| R CG | (2,19)=10.17 | 0.52 | 0.001 | −0.14 | −0.66 | 0.52 | −0.62 | −2.96 | 0.008 | 0.46 | |
| R Precuneus | (2,19)=6.02 | 0.39 | 0.009 | −0.26 | −1.10 | 0.29 | −0.42 | −1.79 | 0.09 | 0.17 | |
| L MFG | (2,19)=12.67 | 0.57 | <0.001 | −0.46 | −2.31 | 0.03 | −0.38 | −1.90 | 0.07 | 0.19 | |
| R Pyramis (cerebellum) |
(2,19)=7.43 | 0.44 | 0.004 | −0.30 | −1.33 | 0.20 | −0.43 | −1.88 | 0.08 | 0.19 | |
| Estradiol (Girls) |
R IOG | (1,17)=17.11 | 0.50 | 0.001 | 0.71 | 4.14 | 0.001 | ||||
IFG = inferior frontal gyrus
IPL = inferior parietal lobule
L = left
MFG = middle frontal gyrus
MOG = middle occipital gyrus
OFC = orbitofrontal cortex
R = right
SFG = superior frontal gyrus
Hierarchical multiple regressions controlling for task performance and movement
Hierarchical multiple regressions controlling for pubertal stage, task performance, and movement
Hierarchical multiple regressions controlling for age
Linear regressions (not controlling for age because lack of age correlation with estradiol)
Hierarchical multiple regressions controlling for age
Cohen’s f2 for hierarchical multiple regressions; small, medium, and large effects: 0.02, 0.15, 0.35 (Cohen, 1988).
Effect sizes for linear regressions (testosterone and estradiol in girls)
Bold = significant main effect or significant main effect after controlling for nuisance variables
3.3 Model 2: Sex Differences in Brain Activity Controlling for Pubertal Stage
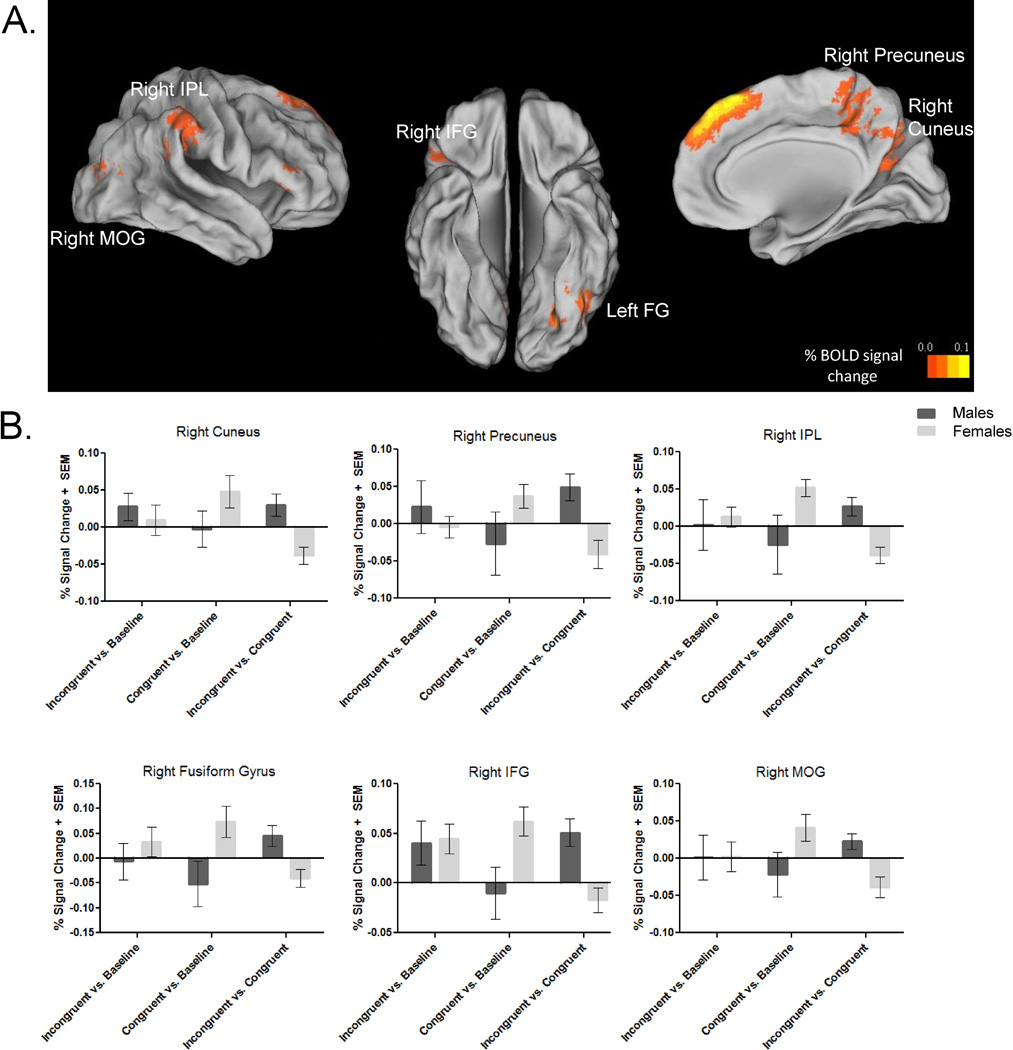
Six clusters of sex differences in Incongruent vs. Congruent brain activity were found to be significant, after controlling for pubertal stage (Figure 4A/Table 5), post-hoc performance, and movement covariates. These clusters were located mainly in occipital and parietal lobules, as well as one region of the prefrontal cortex, with peak coordinates in the right cuneus, right precuneus, right inferior parietal lobule (IPL), left fusiform gyrus, right inferior frontal gyrus (IFG), and right middle occipital gyrus (MOG). In all of these regions, males had heightened emotional conflict-related brain activity relative to females, after controlling for performance and movement nuisance variables (Table 6). Closer examination of simple effects from the Incongruent vs. Congruent contrast suggested that differences in Congruent brain response between males and females were driving these effects. Specifically, in the right IPL, left fusiform gyrus, right IFG, and right MOG, group differences in Congruent vs. baseline (fixation) brain activity were significant or at trend-level, such that females activated these regions significantly more than males, who showed deactivation in these areas (Figure 4B). There were no significant sex-by-puberty interactions in this model. Brain activity between Happy Congruent and Fear Congruent trials, as well as Happy Incongruent and Fear Incongruent trials was compared to confirm that sex differences were not driven by a specific emotional face valence. Paired samples t-tests indicated one significant difference in the right IFG, where Happy Incongruent signal was significantly higher than Fear Incongruent signal (t = 2.46, p = 0.02). A mixed model ANOVA confirmed no significant interaction between sex and Incongruent trial brain activity (F(1,42) = 0.62, MSE = 0.002, p = 0.44).
Figure 4. Sex differences in emotional conflict-related brain activity controlling for pubertal stage.
(A) The effects of sex on emotional conflict-related brain response indicate greater emotional conflict-related brain response in males than females in cuneus, precuneus, inferior parietal lobule, fusiform gyrus, inferior frontal gyrus, and middle occipital gyrus (voxel/clusterwise corrected, p/α < 0.01/0.05, minimum cluster size: 20 voxels). Blood oxygen level-dependent activity maps are surface mapped onto the Population-Average, Landmark-, and Surface-based (PALS-B12) atlas in Talairach space (Van Essen et al., 2001). FG = fusiform gyrus, IFG = inferior frontal gyrus, IPL = inferior parietal lobule, MOG = middle occipital gyrus.
(B) Bar graphs illustrate percent signal change in the blood oxygen level-dependent response for male and female youth. Incongruent vs. Congruent contrasts and each trial type against baseline brain response are plotted. IFG = inferior frontal gyrus, IPL = infrerior parietal lobule, MOG = middle occipital gyrus.
3.4 Model 3: Relationship Between Sex Steroids and Emotional Conflict-related Brain Activity
3.4.1 Testosterone
Testosterone was significantly negatively associated with emotional conflict-related brain response in the left putamen/lentiform nucleus and left MFG in males (p < 0.01/α < 0.05; Figure 5/Table 5). Hierarchical regressions showed that although age was also significantly related to brain activity in these regions (left MFG: F(1,17) = 8.87, R2 = 0.34, p = 0.008; age: β = −0.59, t = −2.98, p = 0.008; left putamen/lentiform nucleus: F(1,17) = 5.38, R2 = 0.24, p = 0.03; age: β = −0.49, t = −2.32, p = 0.03), testosterone explained variance above and beyond the effects of age (Table 6). In females, there were three clusters of emotional conflict-related brain activity significantly associated with testosterone, located in the cerebellum (left and right cerebellar tonsil), and left precuneus. In all three regions, higher testosterone levels were negatively correlated with brain activity. Regressions showed that age was not significantly related to BOLD response in either cluster, so hierarchical regressions were not performed to control for age in these clusters (Table 6).
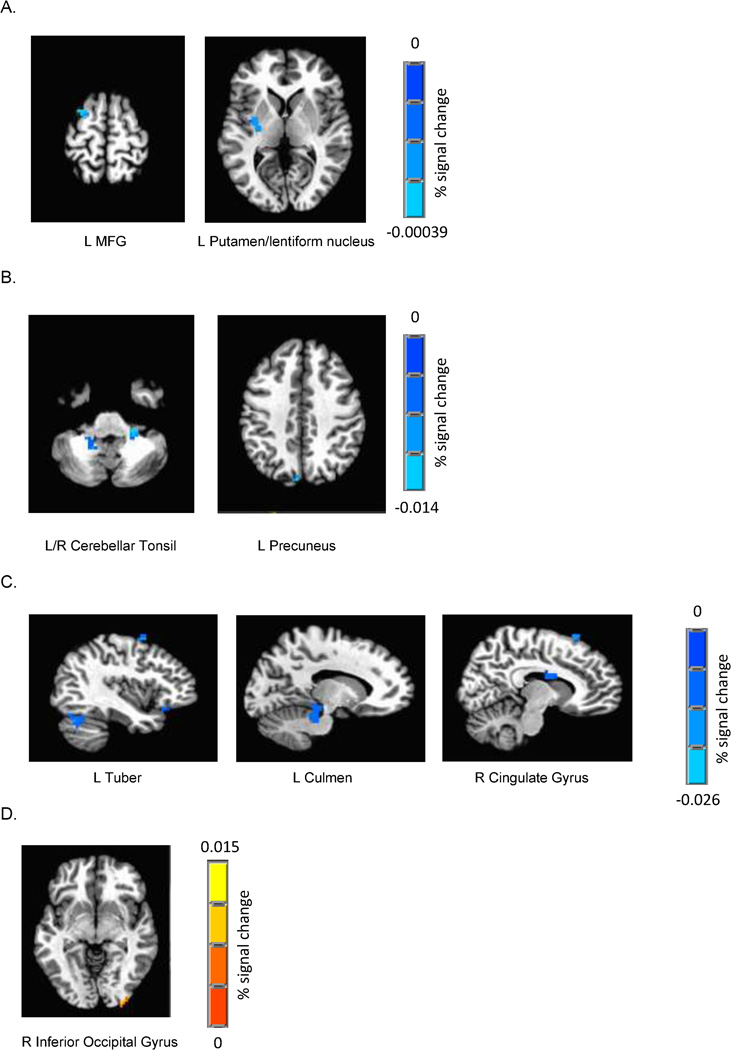
Figure 5. Testosterone and estradiol relationships with emotional conflict-related brain activity.
Significant negative associations (voxel/clusterwise corrected, p/α < 0.01/0.05, minimum cluster size: 13 voxels) between testosterone and emotional conflict-related brain response were found in the left left middle frontal gyrus and the left putamen/lentiform nucleus in males (controlling for age) (A), and in the bilateral cerebellar tonsil and left precuneus in females (voxel/clusterwise corrected, p/α < 0.01/0.05, minimum cluster size: 19 voxels) (B). Significant associations (voxel/clusterwise corrected, p/α < 0.01/0.05, minimum cluster size: 16 voxels) between estradiol and emotional conflict-related brain response were found in the right tuber, left culmen, and right cingulate gyrus in males (controlling for age) (C), and in the right inferior occipital gyrus in females (voxel/clusterwise corrected, p/α < 0.01/0.05, minimum cluster size: 16 voxels). Sagittal and axial views are illustrated with AFNI’s anatomical template as the underlay to display the extent of activation in the clusters. MFG = middle frontal gyrus.
3.4.2 Estradiol
Estradiol was significantly negatively related to brain response in eight clusters in males (p < 0.01/α < 0.05; Figure 5/Table 5). Hierarchical regressions showed that age was also significantly related to brain activity in all eight clusters (left tuber: F(1,20) = 12.29, R2 = 0.38, p = 0.002; age: β = −0.62, t = −3.51, p = 0.002; left SFG: F(1,20) = 25.87, R2 = 0.56, p < 0.001; age: β = −0.75, t = −5.09, p < 0.001; left culmen: F(1,20) = 14.68, R2 = 0.42, p = 0.001; age: β = −0.65, t = −3.83, p = 0.001; left IFG: F(1,20) = 24.59, R2 = 0.55, p < 0.001; age: β = −0.74, t = −4.96, p < 0.001; right cingulate gyrus: F(1,20) = 8.32, R2 = 0.29, p = 0.009; age: β = −0.54, t = −2.88, p = 0.009; right precuneus: F(1,20) = 7.96, R2 = 0.29, p = 0.01, age: β = −0.53, t = −2.82, p = 0.01; left MFG: F(1,20) = 19.23, R2 = 0.49, p < 0.001; age: β = −0.70, t = −4.39, p < 0.001; right pyramis: F(1,20) = 10.04, R2 = 0.33, p = 0.005, age: β = −0.58, t = −3.17, p = 0.005). After adding estradiol to the model, three clusters (two cerebellar regions (tuber and culmen) and the cingulate gyrus cluster) remained, where estradiol explained variance above and beyond age-related effects (Table 6). In the remaining five regions, age alone showed significant contributions to variance in emotional conflict-related brain activity or accounted for enough variance, such that estradiol only contributed at trend level to brain activity in those clusters, once age was accounted for (Table 6). There was one cluster of brain activity significantly positively related to estradiol in females in the right inferior occipital gyrus. A regression showed that age was not significantly related to BOLD response in this cluster, so a hierarchical regression was not performed to control for age in this region (Table 6).
4. Discussion
The goal of the current study was to examine the effects of age, sex, and hormones on emotional conflict-related BOLD response during adolescence. Previous research has explored the effects of age, sex, and hormones on brain response during adolescent development, but to our knowledge, this is the first study to examine each of these variables within the same study. The use of the EmC Task adds to a limited, but growing literature examining concurrent emotional processing and executive functioning during adolescence (Hare et al., 2008; Mincic, 2010; Somerville et al., 2011). This is particularly relevant given that adolescence is a critical developmental period for these systems. As the imbalance between limbic and higher-order cognitive control circuitry may underlie the development of psychopathology during adolescence, more research utilizing tasks that probe the interactions of these systems is necessary.
4.1 Age-related Effects
The results of the current study showed that age related negatively to left and right middle frontal gyrus activity during emotional conflict, even after controlling for performance and movement confounds. Contrary to our hypothesis that age would relate positively to top-down cognitive control brain response, we did not find any positive associations with age and brain response. On the other hand, the current findings suggest that less activity in some frontal regions is associated with greater maturity. There are a few possible explanations for these discrepancies. First, with respect to studies of cognitive control brain activity across adolescence, few have used paradigms with simultaneous emotional and cognitive processing (Hare et al., 2008; Mincic, 2010; Somerville et al., 2011). The developmental maturation of cognitive control abilities has been largely examined using motor (Rubia et al., 2007; Tamm et al., 2002) and oculomotor (Luna et al., 2001) response inhibition tasks, which have primarily found greater (but in some cases reduced) brain activity during inhibition with increased age. Other fMRI studies have used the Stroop task (Adleman et al., 2002; Andrews-Hanna et al., 2011; Marsh et al., 2006), which is similar to the EmC Task, but with a verbal cognitive interference component. With regard to Stroop-related brain activity, studies have found both negative and positive associations with age. For example, Andrews-Hanna and colleagues (2011) found positive relationships between activation and age in the MFG, OFC, temporal lobe, amygdala, and lingual gyrus, but found no negative relationships between age and activation. Similarly, Adleman et al. (2002) found greater frontal, striatal, and parietal brain activity during the Stroop task with increased age, while no negative relationships between age and activity were present. However, Marsh et al. (2006) found significantly lower medial prefrontal cortex, superior temporal gyrus, and parietal lobe activity with increased age, suggesting that older youth may utilize some brain regions less during a cognitive interference paradigm. In fact, as Crone and Dahl (2012) point out, the view of immature prefrontal cortex (PFC) activity during adolescence is difficult to reconcile with mixed findings of both positive and negative activity in different regions of the PFC with age. It is likely that the emotional context of the EmC Task is particularly important to the neural processes that take place during response inhibition. However, we cannot rule out the possibility that these results may have also been present in a task that included non-emotional conflict, since neutral faces were not included in the current EmC Task.
Prior emotion-cognition research paradigms that are most similar to the current study used emotional go-nogo (Hare et al., 2008; Somerville et al., 2011) and emotional Stroop (Mincic, 2010) tasks to examine concurrent affective and cognitive processing to explore the development of these brain systems in healthy youth. While the go-nogo study found that emotional processing was heightened during adolescence, and age interactions with emotional stimuli and gender were explored using a group-level analysis, no linear effects of age were examined. The emotional Stroop study of cognitive interference used a block design task and was conducted in a sample of 16–17 year olds, so trial specific cognitive control could not be extracted and, again, no age-related relationships were examined. The current study is the first to report age-specific effects related to cognitive control during an emotion-cognition task in a developing sample. Less MFG activity with older age could suggest reduced reliance on cognitive processing regions, as a result of increased local efficiency of brain networks that are developing during adolescence (Poldrack, 2014). For example, age-related increases in local efficiency (Wu et al., 2013), or the most direct path between the MFG and its neighbors, could explain why reduced activity of the MFG may be seen with increased age. Other possible explanations for less MFG activity with age could be related to changes in neural energy used by brain regions (e.g. resulting from synaptic pruning) (Poldrack, 2014), and could result in reduced BOLD activity in older relative to younger adolescents when performing the task. Further, in a task of inhibitory control that is more difficult to perform for younger adolescents, factors beyond task performance, such as meta-task difficulty of performing this task in a scanner environment (Poldrack, 2014) could be changing over the course of adolescence. Decreased meta-task difficulty with age could also lead to reductions in frontal brain response in older relative to younger adolescents. Further research will be needed to explore these differing possibilities. The current results suggest that at a group-level, some brain areas are disengaging during emotional conflict across development while adolescents perform this task. It is plausible that at an individual level, ineffective disengagement could represent developmental delays or neural markers of atypical developmental trajectories during emotion-cognition processing, but an appropriate control that examined conflict processing in the presence of neutral faces would be needed to interpret the specificity of these findings to emotional face stimuli.
Furthermore, differences between sample characteristics, task design, and analytic approaches between the current and past studies are worth considering to aid interpretation. The current study was conducted in a sample of 10–15 year olds to more explicitly capture adolescent development and pubertal maturation. Other inhibitory control studies have used a wider age range (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Marsh et al., 2006; Rubia et al., 2007; Tamm et al., 2002) or conducted group analyses between adults, adolescents, and/or children (Adleman et al., 2002; Hare et al., 2008; Somerville et al., 2011). Differences in the tasks used (type of task and whether block or event-related), as well as the possibility of non-linear relationships with age (especially across studies with wider age ranges) may make findings difficult to compare. A strength of the current analytic approach was that we controlled for both task performance and movement-related confounds, often associated with age. Since task performance and age are often correlated, controlling for performance can wash out significant age-related relationships with brain activity (Velanova, Wheeler, & Luna, 2009). However, in the current study, we detect age-related changes that may reflect maturation of local efficiency, neural energetics, or meta-task difficulty, rather than performance-associated changes in brain activity.
Interestingly, we found no significant positive associations in emotional conflict-related brain response with age. This may suggest that the relationship between maturational changes in brain regions and increasing age might not be linear and could be investigated using non-linear analytic strategies. It is also possible that we were unable to detect brain regions which are activated more across development with this restricted age range.
4.2 Sex Differences
While there were no significant sex differences in task performance, such differences in emotional conflict-related brain activity were widespread. In particular, males showed greater activity in visual and parietal cortices compared with females. These findings were significant after controlling for differences in pubertal stage, suggesting sex effects unrelated to pubertal maturation. However, closer examination of simple effects suggested that group differences were driven by the Congruent condition of the task. In most of the significant clusters, males showed statistically significant deactivation during the Congruent condition, while females displayed activation. Males and females did not differ in Incongruent BOLD response. These results suggest that while males are able to disengage visual and parietal attention systems during Congruent trials, females show heightened brain activity to emotional faces during this condition. Visual cortical areas have been previously shown to be activated during emotional arousal (Lang et al., 1998), and gender differences in emotional face processing are present during adolescence (Lee et al., 2013). The allocation of greater visual and attentional resources during Congruent trials in females suggests sex-specific effects on brain response that could explain greater sensitivity to emotional faces in adolescent females (Lee et al., 2013), and may be related to the greater prevalence of internalizing disorders in adolescent females relative to males (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003; Earls, 1987; Hankin et al., 1998).
The lack of expected differences on Incongruent trials could be explained by protracted maturation of brain networks that are engaged by more difficult trials across both males and females. Incongruent trials may be equally taxing at the neural level for adolescent males and females, making it difficult to detect differences in brain activity during these trials. Visual and attentional brain regions involved in brain response during the Congruent trials, could already be in a more mature or stable state, which may allow for sex differences in neural activity to be more readily revealed within this developmental period.
Although, few adolescent fMRI studies have examined sex-specific brain activity, a study relevant to the current paradigm examined sex differences in cognitive control on incongruent vs. congruent trials using the Simon Task (Christakou et al., 2009), and found greater right IFG, right precuneus and right IPL activity in males vs. females, similar to the current findings. However, the direction of the differences is unknown, as simple effects were not reported. Future research will be needed to determine whether these findings replicate across other studies that use cognitive interference paradigms during adolescence.
4.3 Hormone Relationships
At a behavioral level, estradiol showed significant negative relationships with Incongruent and Congruent reaction time in males, and a significant positive relationship with Congruent trial accuracy in females. Faster reaction time with higher estradiol levels in males could be related to previous findings of improved processing speed in males that has been linked with white matter maturation (Turken et al., 2008). Estradiol has shown positive relationships with fractional anisotropy in adolescent males (Herting et al., 2012), so it is plausible that these behavioral findings have an underlying neurobiological correlate, such as increased white matter integrity, which could potentially mediate the relationship between estradiol and processing speed.
The relationship between Congruent trial accuracy and estradiol in females may be related to maturational changes in socio-emotional processing, especially among females during adolescence. Variation in estradiol levels across the menstrual cycle has been shown to relate to emotional processing in females (Derntl, Kryspin-Exner, Fernbach, Moser, & Habel, 2008; Derntl, Windischberger, et al., 2008), such that higher accuracy on emotion recognition is found during the follicular phase of the menstrual cycle. It is possible that higher estradiol levels even within the follicular phase may provide an advantage on emotion recognition for females.
We conducted exploratory analyses to examine whether testosterone and estradiol showed relationships with emotional conflict-related brain response, after controlling for age. Testosterone was negatively related to putamen and MFG activity in males and to cerebellar and precuneus response in females. Estradiol was negatively related to cingulate gyrus and fronto-cerebellar brain activity in males, and positively related to occipital activity in females.
These findings suggest that testosterone has sex-specific relationships with BOLD response during cognitive control. Other developmental studies have also found negative relationships between testosterone and neural characteristics, but most of these have investigated brain structure, not function (Bramen et al., 2012; Bramen et al., 2011; Herting et al., 2012; Neufang et al., 2009; Nguyen et al., 2013). In rats, androgen receptors are weakly to moderately expressed in the forebrain and basal ganglia (Simerly, Chang, Muramatsu, & Swanson, 1990), which could explain relationships between hormone levels and brain response in these areas. Testosterone is thought to be functionally relevant to socio-affective response and influences fronto-striatal-limbic systems (Ladouceur, 2012), so its negative relationship with fronto-striatal brain activity in males could suggest that hormonal maturity in males may be related to maturation of cognitive control in these regions during emotional conflict, key areas implicated in response inhibition (Rubia et al., 2007). However, functional connectivity studies are needed, since a recent adolescent study suggested that higher testosterone can reduce coupling between the orbitofrontal cortex and the amygdala, which could be one pathway for risk of affective disorders (Spielberg et al., 2014).
In females, higher testosterone levels were related to less activity in two clusters of the cerebellum during inhibitory control. Immunohistochemical analysis of 5α-reductase, the enzyme that metabolizes testosterone, shows abundant localization in rat cerebellar Purkinje cells (Castelli et al., 2013), as does the expression of androgen receptors in this layer of cerebellar cortex (Simerly et al., 1990), suggesting preserved function across species. Although speculative, given that testosterone is significantly lower in females vs. males, this hormone may have very different relationships with brain response in the sexes or perhaps the distribution of these sex steroid receptors may be different in human males and females, as has been shown in animal models (Roselli, 1991). Future research is needed to support this explanation.
Further, estradiol’s negative relationship in males with brain activity in cerebellar regions implicated in executive functioning (Schmahmann & Pandya, 1997; Schmahmann & Sherman, 1998) is consistent with other developmental studies that have also found negative relationships between estradiol and neural characteristics, including white matter integrity (Herting et al., 2012) and grey matter density (Peper et al., 2009). To our knowledge, however, this is the first study to report on estradiol’s relationship with brain functioning. Estrogen receptor-β is expressed in the cerebellum (Simerly et al., 1990) and may modulate cognitive functioning by its effects on glutamatergic transmission (Hedges, Ebner, Meisel, & Mermelstein, 2012). However, it is yet to be determined whether estradiol’s negative relationship with cerebellar activity during emotional conflict represents changes in neural energetics or possibly local efficiency among male youth.
The positive association between estradiol and occipital brain activity found in females may suggest estradiol’s modulation of visual-attention brain response in females during emotional conflict in an effort to overcome distraction from emotionally irrelevant stimuli. Interestingly, occipital grey matter volume has been shown to increase across adolescence (Giedd et al., 1999), so it will be important to determine if estradiol levels contribute to changes in occipital brain response, regardless of age. It is clear that more work needs to be done to dissociate the differential effects of sex steroids on brain activity during cognitive, emotional processing, and emotion-cognition paradigms – differences that may in part be due to differential distribution of sex steroid receptors between males and females (Roselli, 1991; Zhang, Cai, Zhou, & Su, 2002; Zuloaga, Zuloaga, Hinds, Carbone, & Handa, 2014) or perinatal organization of brain structure by sex hormones (Arnold & Breedlove, 1985). This avenue of research may help elucidate biological mechanisms that modulate brain maturation and response during development and eventually inform studies of how these mechanisms may contribute to atypical neurodevelopment in child and adolescent psychiatric disorders.
4.4 Limitations
The current study has some limitations that merit discussion. First, the task utilized to examine emotion-cognition interactions did not have conditions in which non-emotional (neutral) faces appeared. Due to this limitation, we cannot conclude that the results of the current study are specific to emotional processing while cognitive interference is present. However, since neutral faces are often considered ambiguous and are processed differently between children and adults (Thomas et al., 2001), it would be difficult to determine what words would be appropriately incongruent for the neutral expressions across individuals. Future studies using the EmC Task may consider overlaying a non-affective, but incongruent word on a neutral face for non-emotional Incongruent trials, and the word neutral on neutral faces for non-emotional Congruent trials, to examine the effect of non-emotional conflict.
Further, while all blood for hormone analyses was collected within a three-hour time window in the morning hours for all participants, due to limitations of participant scheduling, MRI scans were conducted within seven days of the blood draw, but not necessarily on the same day. Thus, hormone values and relationships with BOLD response may have been different had they all been collected the day of the scan, although previous work in adolescents suggests highly correlated sex steroid measurements for both testosterone and estradiol collected on consecutive days (Op de Macks et al., 2011). It should also be noted that while standardization of blood collection was achieved for postmenstrual females, this was not possible for premenstrual females, so greater variability in hormone levels may be present in those youth. However, only values above the detection limit were included in the hormone analyses. If permitting, it would be advantageous for future studies to only include postmenstrual females, to limit any potential variability in hormone levels between them and premenstrual females. Further examination of effect sizes and achieved power for the exploratory hormone analyses suggested that while all effect sizes were medium-to-large (Cohen’s f2 = 0.17–0.56), achieved power for all but one of the significant clusters in the estradiol analyses in males was low (range: 0.45–0.67), and indicated that a larger sample size (≥30 youth) would have been needed to achieve high power (0.80).
In addition, some expected relationships such as sex-by-puberty interactions were not found when controlling for nuisance variables. It should be noted that puberty was assessed with self-report, and not physician reported pubertal stage. This may result in under or overestimated pubertal stages. Also, while puberty was significantly different between males and females, the mean PDS score differences were within one pubertal stage between the groups, which could have made interactions difficult to detect. Further, age-matched, females were more pubertally mature than males in the current study. Another viable approach could have been matching on puberty, yet that analysis would likely have resulted in older males than females, as pubertal maturation tends to occur later in males than females. We acknowledge the difficulty of disentangling these variables in developmental analyses, and suggest careful examination of developmental covariates in any age-by-sex or sex-by-puberty interactions.
Previous publications of the EmC Task have used a trial-by-trial analysis, such that trial types were modeled by taking into account the preceding trial, and thus analyzing brain response to conflict resolution (e.g. previous trial Incongruent, current trial Incongruent) (Etkin et al., 2006). This behavioral effect of increased accuracy on conflict resolution trials relative to Incongruent trials that are preceded by Congruent trials was not present in this sample of adolescents. Due to absence of this behavioral effect, imaging analyses on conflict resolution were not analyzed for the current study.
Finally, the neural correlates of emotional conflict-related brain response may have been different if child faces had been used in the task, as opposed to adult faces. Recent studies are beginning to develop affective facial displays using child faces (Dalrymple, Gomez, & Duchaine, 2013; Egger et al., 2011), so it will be important to examine neural responses to peer emotions, since socio-affective development is critical to adolescent maturity (Goddings et al., 2012).
4.5 Conclusions
The current study found age-related, sex-specific, and hormone-related associations with brain activity during an emotion-cognition task in adolescents. The strength of the study is the assessment of top-down cognitive control in the context of emotional processing during development in a more ecological way than simple motor or verbal tasks allow. Specifically, our findings showed reduced emotional conflict-related brain activity with age in middle frontal gyrus, even after controlling for performance and movement confounds, suggesting changes in utilization of frontal lobe resources across development. Thus, maturation of higher-order cognitive brain regions may be critical in healthy development when top-down control is needed during emotional processing, but deviation from this pattern could represent an atypical trajectory of neural development. We also found sex differences in brain activity during Congruent trials, where females had more activity relative to males, suggesting greater allocation of visual resources in females. This may indicate a bias towards emotional information that may promote affective responding and be a neural risk marker towards internalizing symptoms in females. Finally, testosterone had significant negative relationships with frontal, striatal, (in males), cerebellar and precuneus (in females) activity, while estradiol was negatively associated with emotional conflict-related response in fronto-cerebellar regions in males, and positively related to occipital brain activity in females. Though exploratory, this suggests that hormone levels, above and beyond age, could modulate executive functioning activity during emotion-cognition interactions in both sexes, in regions that underlie cognitive processing. Future research in healthy adolescents should investigate these associations using a longitudinal approach to examine whether these relationships change across late adolescence and to determine whether the current findings have predictive value in determining the emergence of psychopathology over the course of development.
Highlights.
Age inversely relates to frontal lobe activity during emotional conflict.
Increased attentional response is seen in girls during non-conflict trials.
Sex steroids relate to emotional conflict activity in frontal and cerebellar areas.
Acknowledgements
Funding for this study was provided by the Dana Foundation (Nagel), the National Institute on Alcohol Abuse and Alcoholism (R01 AA017664 – Nagel), the National Institute of Mental Health (R01 MH091860 and R21 MH097984 – Etkin), the U.S. Department of Veterans Affairs (Etkin), the Cohen Foundation (Etkin), the Dana Foundation (Etkin), and the Brain Resource (Etkin). We thank Karen Hudson, Emily Maxwell, Kristin Maple, and Nate Spofford for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Alarcon G, Cservenka A, Fair DA, Nagel BJ. Sex differences in the neural substrates of spatial working memory during adolescence are not mediated by endogenous testosterone. Brain Res. 2014;1593:40–54. doi: 10.1016/j.brainres.2014.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS One. 2011;6(6):e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19(4):469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Blanton RE, Cooney RE, Joormann J, Eugene F, Glover GH, Gotlib IH. Pubertal stage and brain anatomy in girls. Neuroscience. 2012;217:105–112. doi: 10.1016/j.neuroscience.2012.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, et al. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS One. 2012;7(3):e33850. doi: 10.1371/journal.pone.0033850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21(3):636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol and Drugs. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J of Adolesc Health. 1993;14(3):190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Castelli MP, Casti A, Casu A, Frau R, Bortolato M, Spiga S, et al. Regional distribution of 5alpha-reductase type 2 in the adult rat brain: an immunohistochemical analysis. Psychoneuroendocrinology. 2013;38(2):281–293. doi: 10.1016/j.psyneuen.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. Sexdependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. Neuroimage. 2009;48(1):223–236. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Corp., I. IBM SPSS Statistics for Windows. Version 20.0. Armonk, NY: IBM Corp; (Released 2011) [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42(6):1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Crockett LJ. Pubertal Development Scale: Pubertal categories. Minneapolis, MN 55455: University of Minnesota Graduate School, 322 Johnston Hall, 101 Pleasant St. SE; 1988. Unpublished manuscript. Copy available upon request from Prof. Anne Petersen, [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Nagel BJ. Risky decision-making: An fMRI study of youth at high risk for alcoholism. Alcoholism: Clinical and Experimental Research. 2012;36(4):604–615. doi: 10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Affect regulation, brain development, and behavioral/emotional health in adolescence. CNS Spectr. 2001;6(1):60–72. doi: 10.1017/s1092852900022884. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21(1):1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Dalrymple KA, Gomez J, Duchaine B. The Dartmouth Database of Children's Faces: Acquisition and Validation of a New Face Stimulus Set. PLoS One. 2013;8(11):e79131. doi: 10.1371/journal.pone.0079131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison M, Whittle S, Yucel M, Vijayakumar N, Kline A, Simmons J, et al. Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Developmental Science. 2013;16(5):772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Derntl B, Kryspin-Exner I, Fernbach E, Moser E, Habel U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Horm Behav. 2008;53(1):90–95. doi: 10.1016/j.yhbeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Lamplmayr E, Kryspin-Exner I, Gur RC, et al. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology. 2008;33(8):1031–1040. doi: 10.1016/j.psyneuen.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand K, Gallay M, Seigneuric A, Robichon F, Baudouin JY. The development of facial emotion recognition: the role of configural information. J Exp Child Psychol. 2007;97(1):14–27. doi: 10.1016/j.jecp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Earls F. Sex differences in psychiatric disorders: origins and developmental influences. Psychiatr Dev. 1987;5(1):1–23. [PubMed] [Google Scholar]
- Egger HL, Pine DS, Nelson E, Leibenluft E, Ernst M, Towbin KE, et al. The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): a new set of children's facial emotion stimuli. Int J Methods Psychiatr Res. 2011;20(3):145–156. doi: 10.1002/mpr.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Ernst M, Korelitz KE. Cerebral maturation in adolescence: behavioral vulnerability. Encephale. 2009;35(Suppl 6):S182–S189. doi: 10.1016/S0013-7006(09)73469-4. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev Neuropsychol. 2011;36(4):429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Maurer D. A happy story: Developmental changes in children's sensitivity to facial expressions of varying intensities. J Exp Child Psychol. 2010;107(2):67–86. doi: 10.1016/j.jecp.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 2012;3(1):19. doi: 10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Burnett Heyes S, Bird G, Viner RM, Blakemore SJ. The relationship between puberty and social emotion processing. Dev Sci. 2012;15(6):801–811. doi: 10.1111/j.1467-7687.2012.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JL, Hamlat EJ, Stange JP, Abramson LY, Alloy LB. Pubertal timing and vulnerabilities to depression in early adolescence: differential pathways to depressive symptoms by sex. J Adolesc. 2014;37(2):165–174. doi: 10.1016/j.adolescence.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107(1):128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges VL, Ebner TJ, Meisel RL, Mermelstein PG. The cerebellum as a target for estrogen action. Front Neuroendocrinol. 2012;33(4):403–411. doi: 10.1016/j.yfrne.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herba CM, Landau S, Russell T, Ecker C, Phillips ML. The development of emotion-processing in children: effects of age, emotion, and intensity. J Child Psychol Psychiatry. 2006;47(11):1098–1106. doi: 10.1111/j.1469-7610.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER. The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI study. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22(9):1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: 1957. [Google Scholar]
- Klapwijk ET, Goddings AL, Burnett Heyes S, Bird G, Viner RM, Blakemore SJ. Increased functional connectivity with puberty in the mentalising network involved in social emotion processing. Horm Behav. 2013;64(2):314–322. doi: 10.1016/j.yhbeh.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD. Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders. Front Integr Neurosci. 2012;6:65. doi: 10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Ryan ND, Casey BJ. Altered emotional processing in pediatric anxiety, depression, and comorbid anxiety-depression. J Abnorm Child Psychol. 2005;33(2):165–177. doi: 10.1007/s10802-005-1825-z. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35(2):199–210. [PubMed] [Google Scholar]
- Lee NC, Krabbendam L, White TP, Meeter M, Banaschewski T, Barker GJ, et al. Do you see what I see? Sex differences in the discrimination of facial emotions during adolescence. Emotion. 2013;13(6):1030–1040. doi: 10.1037/a0033560. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(4):443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The Developmental Mismatch in Structural Brain Maturation during Adolescence. Dev Neurosci. 2014 doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Mincic AM. Neural substrate of the cognitive and emotional interference processing in healthy adolescents. Acta Neurobiol Exp (Wars) 2010;70(4):406–422. doi: 10.55782/ane-2010-1813. [DOI] [PubMed] [Google Scholar]
- Moore WE, 3rd, Pfeifer JH, Masten CL, Mazziotta JC, Iacoboni M, Dapretto M. Facing puberty: associations between pubertal development and neural responses to affective facial displays. Soc Cogn Affect Neurosci. 2012;7(1):35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC. The influence of emotion on cognitive control: relevance for development and adolescent psychopathology. Front Psychol. 2011;2:327. doi: 10.3389/fpsyg.2011.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19(2):464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, et al. Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 2013;23(6):1424–1432. doi: 10.1093/cercor/bhs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Op de Macks ZA, Gunther Moor B, Overgaauw S, Guroglu B, Dahl RE, Crone EA. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Dev Cogn Neurosci. 2011;1(4):506–516. doi: 10.1016/j.dcn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29(38):11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, et al. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34(3):332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth and Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn Sci. 2012;16(6):322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, 3rd, Oswald TM, Mazziotta JC, Iacoboni M, et al. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69(5):1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Is "efficiency" a useful concept in cognitive neuroscience? Dev Cogn Neurosci. 2014 doi: 10.1016/j.dcn.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE. Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology. 1991;128(3):1310–1316. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- Rubia K, Hyde Z, Halari R, Giampietro V, Smith A. Effects of age and sex on developmental neural networks of visual-spatial attention allocation. Neuroimage. 2010;51(2):817–827. doi: 10.1016/j.neuroimage.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Rubia K, Lim L, Ecker C, Halari R, Giampietro V, Simmons A, et al. Effects of age and gender on neural networks of motor response inhibition: From adolescence to mid-adulthood. Neuroimage. 2013;83C:690–703. doi: 10.1016/j.neuroimage.2013.06.078. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schel MA, Crone EA. Development of response inhibition in the context of relevant versus irrelevant emotions. Front Psychol. 2013;4:383. doi: 10.3389/fpsyg.2013.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Menz MM, Miedl SF, et al. Boys do it the right way: sex-dependent amygdala lateralization during face processing in adolescents. Neuroimage. 2011;56(3):1847–1853. doi: 10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc. 2005;11(5):631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Smith AR, Chein J, Steinberg L. Impact of socio-emotional context, brain development, and pubertal maturation on adolescent risk-taking. Horm Behav. 2013;64(2):323–332. doi: 10.1016/j.yhbeh.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, Forbes EE, Ladouceur CD, Worthman CM, Olino TM, Ryan ND, et al. Pubertal testosterone influences threat-related amygdala-orbitofrontal cortex coupling. Soc Cogn Affect Neurosci. 2014 doi: 10.1093/scan/nsu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Dev Rev. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar Stereotaxic Atlas of the Human Brain. Three-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme; 1988. [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, et al. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Casey BJ. Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Front Psychol. 2011;2:39. doi: 10.3389/fpsyg.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. Journal of the American Medical Informatics Association : JAMIA. 2001;8(5):443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J Neurosci. 2009;29(40):12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman I, Toni I, Verhagen L, Roelofs K. Endogenous testosterone modulates prefrontal-amygdala connectivity during social emotional behavior. Cereb Cortex. 2011;21(10):2282–2290. doi: 10.1093/cercor/bhr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corp; 1999. [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35(1):205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Hashizume H, Sassa Y, Takeuchi H, et al. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PLoS One. 2013;8(2):e55347. doi: 10.1371/journal.pone.0055347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JQ, Cai WQ, Zhou DS, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935(1–2):73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL, Handa RJ. Estrogen receptor beta expression in the mouse forebrain: age and sex differences. J Comp Neurol. 2014;522(2):358–371. doi: 10.1002/cne.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]