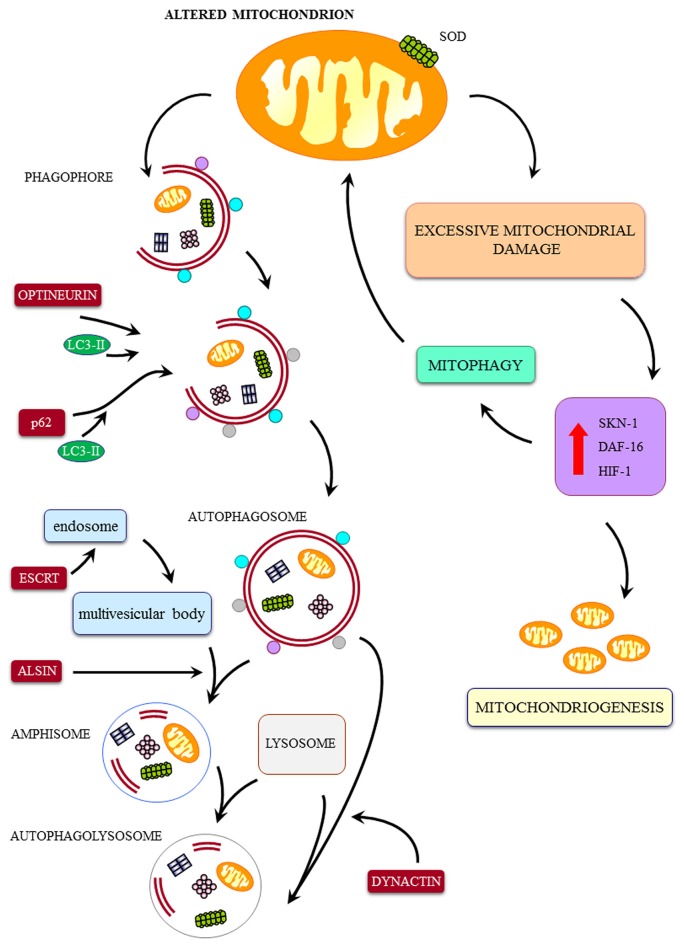
Figure 2.
Cartoon on the major pathways involved in mitochondrial integrity and a few examples of ALS-related alterations. The mitochondrial dysfunctions in ALS may be produced by a direct mitochondrial toxicity (exemplified here by SOD1-induced mitochondrial toxicity) or a defect in the removal of altered mitochondria by the autophagy/mitophagy pathway. These include: (1) defect in the merging of autophagosome with lysosome (dynactin mutation); (2) defect of merging of endosome with autophagosome to produce amphisome (alsin mutation); (3) defect in linking ubiquitinated protein aggregates to the autophagy machinery by the autophagy protein p62 (SQSTM1 mutation); (4) defect of the fusion of autophagosomes with endosomes and lysosomes (CHMP2B mutation); (5) defect in vesicles trafficking beyond the autophagosome (dynactin mutation); (6) defect in parkin-mediated mitophagy (Optineurin mutation); (7) defect in autophagosome maturation and mitophagy (VCP mutation); and (8) defect in trafficking of autophagy compartments (C9orf72 mutation). Despite a sole defect in the biogenesis of mitochondria may potentially lead to accumulation of degenerated mitochondria, to our knowledge a specific familial ALS (fALS) phenotype due to such a defect was not described so far. Nonetheless, it is likely that, due to a dual tightened control of mitochondrial removal and biogenesis of mitochondria, a failure in the first pathway will eventually lead to a failure in the biogenesis of novel mitochondria. Thus, it is not surprising that, in all fALS phenotypes featuring a defect in the progression of autophagy, we can detect only giant, altered mitochondria in the absence of small, newly synthesized mitochondria. This confirms the eventual concomitance of mitophagy and mitochondriogenesis as indicated by Palikaras et al. (2015a,b). Degenerated mitochondria, to our knowledge a specific fALS phenotype due to such a defect was not described so far. Nonetheless, it is likely that, due to a dual tightened control of mitochondrial removal and biogenesis of mitochondria, a failure in the first pathway will eventually lead to a failure in the biogenesis of novel mitochondria. Thus, it is not surprising that, in all fALS phenotypes featuring a defect in the progression of autophagy, we can detect only giant, altered mitochondria in the absence of small, newly synthesized mitochondria. This confirms the eventual concomitance of mitophagy and mitochondriogenesis as indicated by Palikaras et al. (2015a,b).