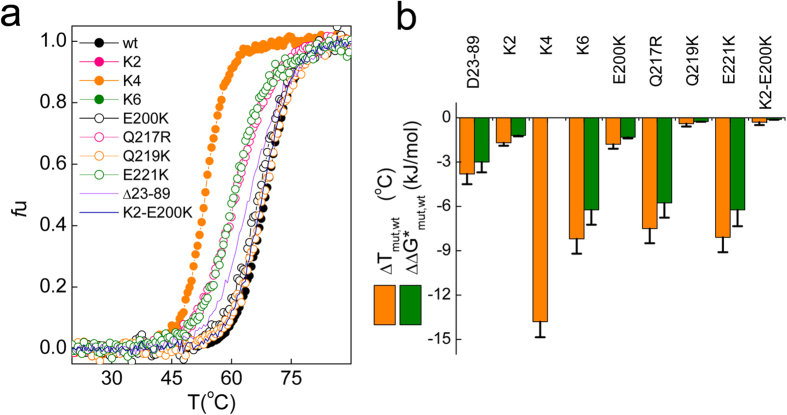
Figure 3. Effect of domain charges on the PrP stability.
(a) Thermal denaturation of PrP wt and mutants. The unfolded fraction was calculated using the ϴ222 temperature function according to a two-state transition, as described48. (b) Differences in the unfolding temperature (ΔTm) and in the free energy of unfolding (ΔΔG*) induced by the charge mutations. ΔTm is the difference between the denaturation temperature of PrP mutant (PrPmut or PrP90−231 wild type) and full length PrPwt (Tmmut −Tmwt). Tm values were obtained as the midpoints of the temperature denaturation curves. ΔΔG* was obtained as ΔH,wt × (1−Tmwt/Tmmut), where ΔHvH is the van´t Hoff enthalpy of PrP wt denaturation (255 kJ/mol). ΔΔG* < 0 indicates a destabilization of the mutant chain compared to the wt. Displayed data are the average of three independent measurements, performed with at least two different protein batches. Error bar represents the standard deviation (s.d.). Calculations for K4 were omitted given its irreversible denaturation.