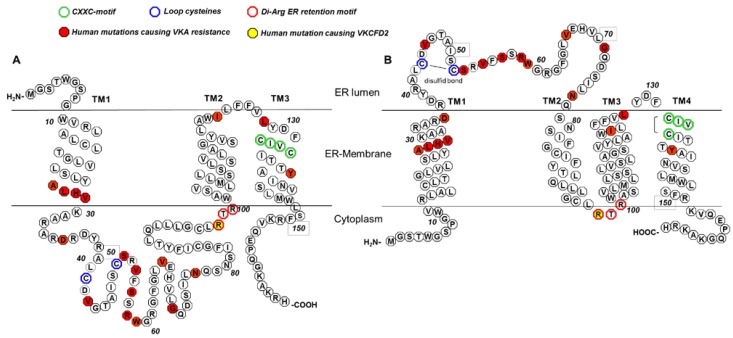
Figure 1.
3TM and 4TM topological models for hVKORC1 (modified from Tie et al., 2012 [26]). In both models, conserved cysteines (Cys132 and Cys135 of the active center (CXXC motif), green; loop cysteines Cys43 and Cys51, blue) and Arg98_Arg100 of the di-arginine endoplasmic reticulum (ER) retention motif (red) are labeled with colored circles. Amino acid positions for which mutations were reported to be associated with either vitamin K antagonist (VKA) resistance or combined deficiency of vitamin K dependent clotting factors type 2 (VKCFD2) are marked by filled circles (mutations causing VKA resistance, red; VKCFD2 mutation, yellow). (A) Shows the putative topology for hVKORC1 as a 3 TM membrane-embedded protein with the loop located in the cytoplasm. The N-terminus is located in the ER lumen, whereas the C-terminus is in the cytoplasm. (B) Shows the putative 4TM topology for hVKORC1 with the loop containing the conserved cysteines Cys43 and Cys51 in the ER lumen with both termini located in the cytoplasm.