Abstract
Background: Expression of SOX-2 and Oct4 as markers for the identification of cancer stem cells (CSCs) has been revealed in several malignancies. In this study, the co-expression of SOX-2 and Oct4 and their correlation with clinicopathological features of endometrial adenocarcinomas (EACs) was investigated. Methods: SOX-2 and Oct4 expression was assessed by immunohistochemistry in 27 (39.13%) stage IA and in 42 (60.87%) stage IB International Federation of Gynaecology and Obstetrics (FIGO) EACs and related to the clinicopathological features of patients. Results: The expression of SOX-2 was confirmed in 62/69 tumour specimens compared to Oct4 expression in 46/69 specimens (P = 0.015) and no difference in median staining intensity between SOX-2 and Oct-4 was observed. The highest median SOX-2 expression was found in high-grade (G3) EAC samples compared to moderate-grade (G2) EAC specimens (P = 0.020) and low-grade (G1) specimens (P = 0.008), while no differences in median Oct4 expression in EAC samples according to grading were present. In G3 specimens, significantly higher median SOX-2 expression was noted compared to Oct4 (P = 0.002). SOX-2 and Oct4 co-expression was observed only in G1 EAC (R: 0.51; P = 0.031). Age of EAC diagnosis was positively correlated with SOX-2 expression (b = 0.193; R2 = 10.83%; P = 0.003) but not to age of menarche, menopause, parity or body mass index. Conclusions: There is no need to use SOX-2 expression as a poor outcome predictor in stage I EAC, and SOX-2 expression should be analysed in more advanced stages.
Keywords: Cancer stem cells, endometrial adenocarcinoma, immunohistochemistry, Oct4, SOX-2
Introduction
Cancer of the endometrium is the most common gynaecologic malignancy in developed countries and the second most common in the developing world, where cervical cancer remains predominant [1]. Endometrioid carcinoma is the common site and histologic subtype of endometrial carcinoma and of uterine cancer overall. Endometrial cancer is characterized by a heterogeneous population of cancer cells surrounded by stroma and a subpopulation of cells exhibiting features of cancer stem cells (CSCs) [2,3]. As carcinogenic mutations can be acquired over many years, it is likely that only adult stem/progenitor cells have a lifespan sufficiently long enough to accumulate the genetic damage necessary to give rise to a cancer. Therefore, CSCs have been hypothesised to be responsible for carcinoma infiltration [2,3]. Recent studies have revealed the critical role of CSCs in tumourigenicity and metastasis. Although representing only a small proportion of the tumour, CSCs are believed to constitute a reservoir of cancer-initiating cells called tumour-propagating cells [2]. To date, CSCs have been identified in numerous solid cancers such as neuroblastoma, breast, colon and lung cancer [4].
In recent years, many studies have demonstrated that aberrant expression of certain stem cell-associated nuclear transcription factors, such as Octamer binding transcription factor 4 (Oct4), Sex-Z, Nanog and Kruppel-like factor 4 (Klf4), could contribute to the tumourogenesis of various somatic cancers [3,5,6]. Sex-determining region y (SRY)-Box2 (SOX2) is a member of the SOX family of transcription factors responsible for coordinating disparate functions such as maintaining stem cell properties and differentiation restriction [7,8]. In particular, SOX2 is involved in the regulation of stem cell destination during embryonic development and its expression level is tightly regulated to ensure normal embryonic development [9]. SOX2 depletion by RNA interference promotes embryonic stem cell differentiation into multiple cell types [10]. SOX2 is a key factor capable of inducing pluripotency in somatic cells along with KLF4, Oct3/4, and c-Myc. It is also one of four transcription factors capable of reprogramming human somatic cells into pluripotent stem cells with characteristics of embryonic stem cells [8,11].
Oct4 is a nuclear transcription factor of the POU-homeodomain family that plays a critical role in several aspects of ESC maintenance including ESC self-renewal, pluripotency and lineage commitment [6-8]. At the top of the primitive pluripotent cell genetic regulatory network, Oct4 and SOX-2 function cooperatively to stimulate the transcription of several target genes including Nanog, FGF-4, UTFl, Fbx15, microRNA-302 clusters and even SOX-2 and Oct4 themselves [12,13]. Consistent with their roles in maintaining pluripotency, overexpression of specific transcription factors (Oct4, SOX-2, KLF4 and c-Myc) can induce somatic cells to acquire pluripotency. These induced pluripotent stem cells have characteristics similar to ESCs [16]. Oct4 may act as a multi-functional factor during cancer development, and ectopic Oct4 expression in somatic cells causes epithelial dysplasia [14,15]. However, to date, no study has defined a potential function for SOX-2 in endometrial cancer. In the present study, we used immunohistochemistry (IHC) to evaluate stem cell markers of SOX-2 and Oct4 expression in 69 early-stage EAC specimens. The co-expression of SOX-2 and Oct4 and their correlation with clinocopathological features was also assessed.
Materials and methods
Patients and specimens
Between January 1 and December 31, 2014, 95 patients were diagnosed with endometrial adenocarcinoma (EAC) after dilation and curettage (D&C) or hysteroscopy procedures in the gynaecology and oncology department at Jagiellonian University (Kraków, Poland). Every patient with initially diagnosed EAC underwent a pelvic examination by a gynaecologic oncologist, pelvic magnetic resonance imaging (MRI) or transvaginal ultrasound examination (TUS), abdominal ultrasonography (AUS) and chest X-ray to determine optimal treatment. There were 69 patients who met the following inclusion criteria: age ≥ 18 years, EAC stage I confirmed by histopathological examination, no hormonal treatment during the 6 months prior to EAC diagnosis. This study was approved by the Ethics Board of Jagiellonian University and all of the included patients provided written informed consent. All included women were primarily treated with surgery with 38 cases undergoing total laparoscopic hysterectomy (TLH) with bilateral salphingooopherectomy (BSO) and pelvic and periaortal lymph node sampling (PALNS) and 31 cases who underwent total abdominal hysterectomy (TAH) with BSO and PALNS. All of the procedures were performed according to European Society for Gynaecological Oncology (ESGO) guidelines. Clinical data of each patient were recorded and included age, parity, menopausal status, previous hysterectomy, personal and familial history of malignancy. Two board-certified pathologists evaluated hematoxylin and eosin (H&E) stained slides for all of the patients to make a final diagnosis and provide pathological staging of the disease according to pathological tumour-node-metastasis (pTMN) classification.
Immunohistochemistry and scoring
Immunohistochemistry was performed on tissue sections (4-5 μm thick) using the same protocol for Sox2 (D6D9 XP, Rabbit mAb. #3579S) and Oct-4A (C52G3, Rabbit mAb, #2890S) obtained from Cell Signalling Technology (Wirginia, US). Slides were deparaffinized and dehydrated in 100% ethanol, washed in distilled water and microwaved (600 W for 10 min and 5 min) in antigen-retrieval solution (citrate buffer, pH 6.0). Then they were washed in distilled water, cooled at room temperature (RT) for 20-30 min and immersed in 3% H2O2 to block endogenous peroxidase after washing in distilled water (5 min) and wash buffer (Tris/HCl, DakoCytomation, S3006) twice for 5 min each. Primary antibodies (100 µL) were applied to each tissue section. The antibodies were diluted 1:100 in Dako Antibody Diluent with Background Reducing Components (S3022) and incubated overnight at 4°C in a humidified chamber. The slides were washed the next day in wash buffer and the secondary antibody was incubated for 75 min at RT (Dako Real EnVision HRP Rabbit/Mouse, K5007). The enzymatic reaction was performed with DAB incubation for 5-10 min at RT. Tissue sections were counterstained with hematoxylin and coverslipped. All of the specimens were positive for SOX-2 and Oct4 nuclear staining. In addition, unspecific cytoplasmic staining for Oct-4 was observed (Figure 1). For the negative control, the same specimen and method were used without the primary antibody.
Figure 1.
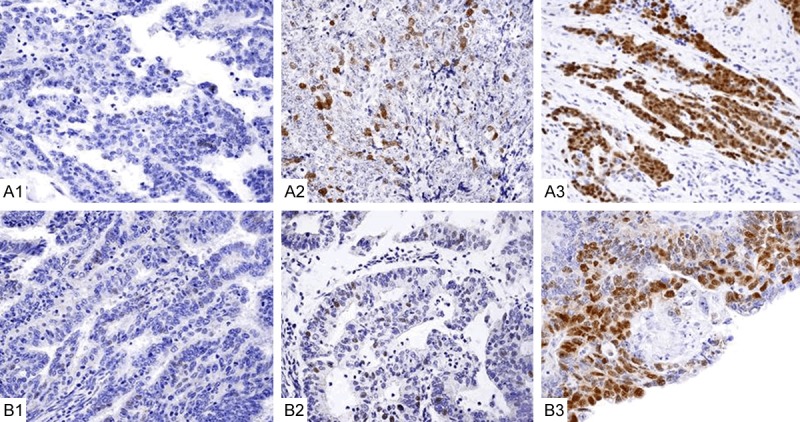
SOX-2 and Oct immunohistochemical staining in early-stage endometrial adenocarcinoma (EAC) quantified as low (+), moderate (++) or strong (+++) staining in low-grade (G1), moderate-grade (G2) and high-grade (G3) tumours. A1: G1. SOX-2 (+) nuclear staining; A2: G2. SOX-2 (++) nuclear staining; A3: G3. SOX-2 (+++) nuclear staining. B1: G1. Oct4 (+) nuclear staining; B2: G2. Oct4 (++) nuclear staining; B3: G3. Oct4 (+++) nuclear staining.
SOX-2 and Oct4 nuclear staining for each slide was evaluated blindly by two board-certified histopathologists in 5 high-power fields (× 40) of maximal staining intensity, so-called “hot spots”. Every tumour was scored according to the intensity of nuclear staining (0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining) and the number of stained cells (0, expression in < 1%; 1, 1-5%; 2, 6-10%; 3, 11-25%; 4, 26-50% and 5, > 50% of cells). In case of disagreements in scoring, the slides were revised until a consensus was obtained, which happened in two cases. The final immunoreactivity score was determined by multiplying the intensity scores by the extent of positivity scores of stained cells, which provided a score range of 0-12.
Statistical analysis
The Shapiro-Wilk test was used to examine the distribution of variables in the patients. Clinical features of the study group and control group were compared using parametric Student’s t-test and non-parametric Mann-Whitney U tests or χ2 tests as appropriate. To compare SOC-2 and Oct4 immunoreactivity, the Wilcoxon-paired test was chosen. The one-way analysis of variance (ANOVA) Kruskal-Willis test was used to evaluate more than two groups of variables followed by the Fisher post-hoc test as appropriate. Multivariate analysis of variance (MANOVA) was used to identify factors that might influence SOX-2 and Oct4 expression. Gamma correlation or multivariate regression was used to evaluate the relationship between SOX-2 and Oct4 expression and clinical features expressed as continuous variables. The clinical features of the study patients are presented as median values and standard deviation (SD) or number of cases and percentage. SOX-2 and Oct4 immunoreactivity were presented using an arbitrary relative scale (points) as median and interquartile range (IQR). P = 0.05 was accepted as statistically significant. All calculations were carried out with the STATISTICA software v. 10 (StatSoft, USA, 2011).
Results
The average age of cancer diagnosis was 60.74 ± 11.60 years; detailed clinical characteristics are presented in Table 1. All of the 69 patients were diagnosed with EAC: 27 (39.13%) in stage IA and 42 (60.87%) in stage IB according to the International Federation of Gynaecology and Obstetrics (FIGO). As none of the patients had lymph node involvement or distant metastases, pTNM classification revealed that 27 (39.13%) cases were T1AN0M0 stage and 42 (60.87%) cases were pT1BN0M0 stage. Most of the patients were diagnosed with moderate-grade (G2) and high-grade (G3) disease (25 [36.23%] and 26 [37.68%]), while only 18 (26.09%) had low-grade (G1) tumours. The most common comorbidities were obesity (49 patients [70.01%]); hypertension (39 patients [56.52%]) and diabetes type I or II (24 patients [34.78%]). There were no significant differences in median body mass index (values when comparing diabetic and non-diabetic patients (30.28 kg/m2 ± 6.17 vs. 26.81 kg/m2 ± 5.51; P = 0.020); however, in 45 non-diabetic patients there were 27 who suffered from obesity. We also did not find any differences in tumour grading distribution between diabetic and non-diabetic (diabetic G1/G2/G3: 7/6/11 vs. non-diabetic G1/G2/G3: 11/19/15; P = 0.542) nor between obese and non-obese women (obese G1/G2/G3: 13/18/16 vs. non-obese: G1/G2/G3: 5/17/8; P = 0.987). There were two patients who were underweight.
Table 1.
Clinical characteristics of patients
| Clinical features | ||
|---|---|---|
| 1 | Age [years] (mean ± SD) | 60.74 ± 11.60 |
| 2 | Age of first period [years] (mean ± SD) | 12.68 ± 1.91 |
| 3 | Age of menopause [years] (mean ± SD) | 50.74 ± 4.35 |
| 4 | BMI [kg/m2] (mean ± SD) | 28.04 ± 6.94 |
| 5 | Menstrual bleeding | |
| Heavy | 18 (26.08%) | |
| Normal | 48 (69.57%) | |
| Scant | 3 (4.35%) | |
| 6 | Painful periods | |
| Yes/No | 19 (27.54%)/50 (75.46%) | |
| 7 | Periods | |
| Regular/Irregular | 62 (89.86%)/7 (10.14%) | |
| 5 | No of pregnancies (median; IQR) | 2; 1.50 |
| 6 | No of deliveries (median; IQR) | 2; 1.00 |
| 7 | Co-morbidities | |
| Hypertension | 67 (97.10%) | |
| Circulatory insufficiency | 5 (7.25%) | |
| Diabetes mellitus | 54 (78.26%) | |
| Hyperthyroidism | 3 (4.35%) | |
| Hypothyroidism | 9 (13.04%) | |
| Asthma | 14 (20.29%) |
IQR, interquartile range; SD, standard deviation.
Expression of SOX-2 and Oct4 in tumour tissues
Most (62/69) of the EAC specimens were immunopositive for SOX-2 protein, while significantly fewer samples were positive for Oct4 (46/69) (P = 0.015). There was no difference in median staining intensity between SOX-2 and Oct4 in the analysed specimens ([1.00; IQR: 1.00 vs. 1.00; IQR: 1.00] P = 0.359) nor in median quantification of the number of positive cells for SOX-2 staining compared to Oct4 staining ([0.02; IQR: 0.15 vs. 0.02; IQR:0.06] P = 0.218) (Figure 1).
Association of SOX-2 and Oct expression with clinicopathological features
Multivariate regression analysis confirmed a significant association between SOX-2 expression and grading (P < 0.001), while there was no relationship between SOX-2 expression and staging, parity or co-morbidities (Table 2). Age of EAC diagnosis was an independent factor positively influencing SOX-2 expression (b = 0.193; R2 = 10.83%; P = 0.003) in a multiple regression model, while age, first or last period and body mass indexI on SOX-2 tumour expression had no effects. Moreover, no relationship between Oct4 expression and tumour staging, grading or parity was indicated. Patient age at EAC diagnosis, age of first period and menopause or body mass index were not correlated with Oct4 expression. The presence of co-morbidities such as type I and II diabetes and obesity were not associated with SOX-2 or Oct4 expression. However, patients with hypertension had significantly higher Oct4 levels compared to normotensive controls (P =0.022) (Table 2). A similar lack of correlation was observed for SOX-2 expression (Table 2).
Table 2.
SOX-2 and Oct4 expression according to clinicopathological features of EAC
| Features | N | SOX-2 expression [median; IQR] | P | Oct4 expression [median; IQR] | P |
|---|---|---|---|---|---|
| Histological grade | |||||
| G1 | 18 | 1.00; 2.00 | < 0.001* | 1.00; 2.00 | 0.910 |
| G2 | 25 | 1.00; 3.00 | 1.00; 4.00 | ||
| G3 | 26 | 6.00; 10.00 | 1.00; 3.00 | ||
| FIGO stage | |||||
| IA | 27 | 2.00; 6.00 | 0.484 | 1.00; 2.00 | 0.263 |
| IB | 42 | 1.50; 6.00 | 1.00; 4.00 | ||
| Parity | |||||
| Nulliparous | 10 | 1.00; 3.00 | 0.759 | 0.50; 2.00 | 0.340 |
| Primiparous | 11 | 2.00; 6.00 | 1.00; 3.00 | ||
| Multiparous | 48 | 1.50; 8.00 | 1.00; 4.00 | ||
| Diabetes | |||||
| YES | 24 | 3.00; 9.50 | 0.097 | 1.00; 4.00 | 0.861 |
| NO | 45 | 1.00; 4.00 | 1.00; 4.00 | ||
| Arterial hypertension | |||||
| YES | 37 | 1.00; 6.00 | 0.698 | 1.00; 2.00 | 0.022* |
| NO | 32 | 2.00; 6.00 | 2.00; 3.00 | ||
| Obesity | |||||
| YES | 47 | 1.00; 6.00 | 0.741 | 1.00; 4.00 | 0.388 |
| NO | 22 | 2.50; 6.00 | 1.00; 4.00 |
EAC, endometrial adenocarcinoma; IQR, interquartile range.
statistically significent value.
The highest median SOX-2 expression was found in G3 EAC samples compared to G2 EAC cases and G1 specimens (P = 0.002) (Figure 2; Table 2). No differences in median Oct4 expression in EAC samples according to grading were present (Figure 2; Table 2). There were also no differences in SOX-2 and Oct-4 expression in women with IA and IB stage disease (Table 2).
Figure 2.
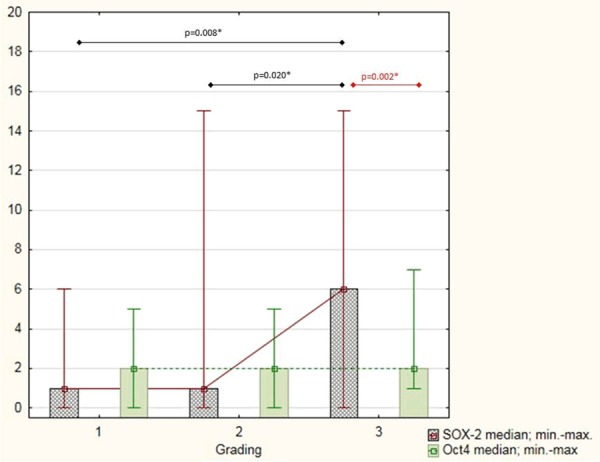
SOX-2 and Oct4 immunohistochemical score based on tumour grading. *statistically significent value.
SOX-2 and OCT4 co-expression in tumour specimens
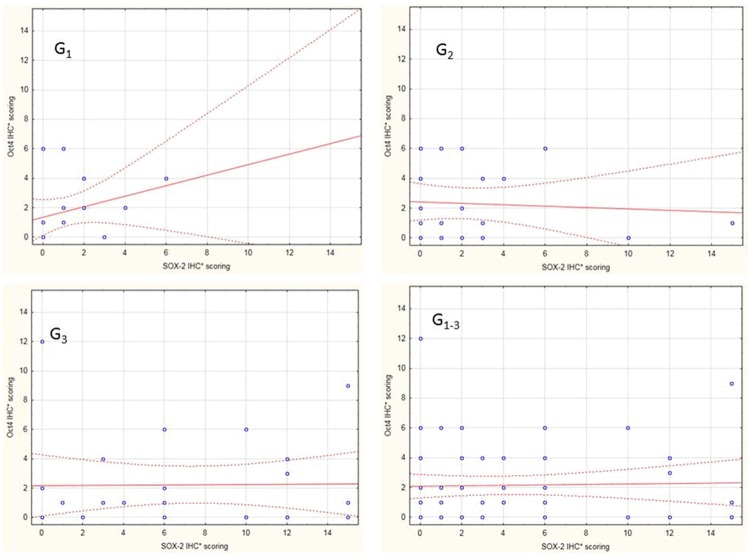
We did not note any differences in co-expression of SOX-2 and Oct4 in the 69 patients overall. However, when patients were classified by tumour grade, a positive significant correlation between SOX-2 and Oct4 was observed in patients with low-grade disease (R: 0.51; P = 0.031) but not in G2 and G3 tumours (Figure 3). Moreover, there was significantly higher median SOX-2 expression in G3 tumours compared to median Oct4 levels ([6.00; IQR 10.00 vs. 1.00; IQR: 3.00]; P = 0.002) (Table 3).
Figure 3.
SOX-2 and Oct4 co-expression in low-grade (G1), moderate-grade (G2) and high-grade (G3) tumours, as well as in the total (G1-3) sample. *IHC-immunohistochemistry.
Table 3.
Multivariate analysis of variance (MANOVA) of SOX-2 and OCT4 expressionaccording to tumour grading
| Low grade EAC N = 18 | Moderate grade EAC N = 25 | High grade EAC N = 26 | P | |
|---|---|---|---|---|
| SOX-2 (median; IQR) | 1.00; 2.00 | 1.00; 3.00 | 6.00; 10.00 | 0.002* |
| OCT4 (median; IQR) | 1.00; 2.00 | 1.00; 4.00 | 1.00; 3.00 | NS |
| P | NS** | NS** | 0.002 |
EAC, endometrial adenocarcinoma; IQR, interquartile range.
statistically significant values;
NS, not significant values.
Discussion
CSCs constitute between 0.2% and 1.2% of the whole population of mammary neoplastic cell lines but play a key role in enhancing tumourogenesis via promotion of cell proliferation, self-renewal and unlimited differentiation [16,17]. SOX-2 and Oct4 are important transcription factors that contribute to both tumour metastasis and chemoresistance [18]. SOX-2 and Oct4 expression have been confirmed in haematological cancers and solid tumours, including gynaecological cancers such as ovarian, endometrial and cervical cancer, as well as breast cancer [19]. Skidan and Steiniger [20] proposed that future anti-cancer therapy should target these cells via chemical compounds, small molecular agents and micro-RNA-based therapeutics. Implementation of tailored therapies against CSCs requires detailed identification of specific markers. However, little is known about SOX-2 and Oct4 expression and their relationship to clinicopathological features in early-stage EAC.
In the present study, the presence of SOX-2- and Oct 4-positive cells in EAC during the early stages of disease was confirmed. We observed predominantly nuclear staining for both SOX-2 and Oct4 in EAC specimens with weak cytoplasm markers. These findings are in contrast with a previous study that confirmed a nucleo-cytoplasmic staining pattern of SOX-2 in bladder cancer [21]. However, high levels of nuclear expression of both SOX-2 and Oct4 have been observed in cervical cancer [22], small cell lung cancer [23] and squamous cell oesophageal cancer, which is consistent with our findings in EAC [24].
It is generally known that patients suffering from solid tumours with SOX-2 overexpression have significantly poorer outcomes [21,22,24]. In our study, the expression of SOX-2, but not Oct4, was positively related to tumour grading. These results are consistent with the findings of other investigators who confirmed SOX-2 overexpression in high-grade breast, bladder, colon and lung cancer [21,23,25,26]. Low-differentiated EAC presents with higher SOX-2 expression compared to well-differentiated tumours even in the early stages of disease, which might increase the risk for local recurrence because SOX-2 overexpression is generally considered to be a poor prognostic marker for recurrence and overall survival. Saigusa et al. [23] demonstrated that patients suffering from small cell lung cancer with SOX-2 overexpression were more often diagnosed with advanced stages of the disease and lymph node metastases. Ruan et al. [21] determined that bladder cancer patients with high expression levels of SOX-2 had significantly worse recurrence-free survival time compared to control patients at the same stage of disease. Moreover SOX-2 expression was positively correlated with tumour size and grade in bladder cancer. In patients with squamous oesophageal cancer, both SOX-2 and Oct4 overexpression were related to significantly shorter overall survival rate compared to patients with negative or weakly stained tumours [24]. SOX-2 overexpression is directly related to lymph node metastases in breast cancer (27). There is a higher risk of distant recurrence in rectal cancer if SOX-2 and Oct4 are overexpressed in the primary tumour [28]. As our study was limited to early-stage EAC without lymph node involvement, analysis of distant metastases or recurrence was not possible. Currently, we know that G3 early EACs have a significantly higher potential to recur and metastasize compared to low-grade tumours, and that SOX-2 overexpression is typically seen in high-grade cancers. Nevertheless, the utility of SOX-2 overexpression in early-stage EAC as a negative marker is very limited from the clinical point of view because it is closely correlated with tumour grading, which is an established pathological prognostic marker.
Co-expression of SOX-2 and Oct4 has been confirmed in several studies of solid tumours [19,24,28]. Wang et al. [24] showed a positive correlation between SOX-2 and Oct4 staining in oesophageal squamous cancer. Positive associations between SOX-2 and Oct4 have also been confirmed in seminoma, colorectal, bladder and lung cancer [19,28]. Consistent with a previous report [19], we did not find co-expression of SOX-2 and Oct4 in EAC tissues. A similar lack of relationship between SOX-2 and Oct4 has been observed in cervical squamous cancer [22]. However, inconsistent results regarding the relationship between SOX-2 and Oct4 in different tumours forced us to conduct supplementary detailed analyses of additional clinical and pathological features. We determined a significant correlation between SOX-2 and Oct4 expression only in low-grade EAC tumours, while no such association was observed in G2 and G3 subgroups or in the total group. With increased tumour grading, we observed continued SOX-2 overexpression, while Oct4 expression remained unchanged. In the vast majority of solid tumours, SOX-2 expression differed significantly according to tumour grading while only few cancers presented grading-dependent Oct4 expression. In breast cancer, positive SOX-2 expression was associated with high tumour grade [27], and similarly, in squamous cervical cancer SOX-2 but not Oct4 was positively correlated with grading [22]. In non-gynaecological malignancies, a positive association between SOX-2 and grade has been demonstrated for bladder, brain, colon, renal, thyroid and head and neck cancers, as well as sarcomas, whereas a positive correlation between Oct4 expression and grading has only been seen in sarcomas, brain, bladder and head and neck cancers [19]. Thus SOX-2 overexpression in high-grade tumours is not only limited to EAC or gynaecological cancers but is also observed in many other malignancies, suggesting an established role for SOX-2 in carcinogenesis that maintains the cell proliferation potential.
Early menarche, late menopause, nulliparity, obesity and impaired glucose metabolism are closely related to an increased risk of EAC. However, when we analysed clinical features with respect to SOX-2 and Oct4 expression, we could not determine any significant relationship in early-stage EAC. In fact, a weak, albeit significant, positive correlation was noted between patient age and SOX-2, but not Oct4, expression was observed. Similarly, SOX-2 expression is not correlated with clinical features including smoking status in patients with small cell lung cancer [23]. We also found that Oct4 expression was not related to age, although EAC patients suffering with hypertension had significantly higher Oct4 expression levels compared to normotensive patients with EAC. These findings require further investigation. A relationship between SOX-2 or Oct4 has not been demonstrated in a vast majority of solid tumours in relation to age, gender, and tumour localization [19,21,23,24]. Thus, the results of our study on EAC clinical features in relation to SOX-2 and Oct expression are consistent with the literature, confirming no utility of SOX-2 and Oct4 as screening markers.
It should be noted that these results are limited to early-stage EAC and must be interpreted with some caution. The main limits of our study include a lack of recurrence and survival data. However, it must be emphasized that the study focused on early-stage EAC, which has the highest overall survival rate and progression-free period compared to other gynaecological malignancies. Moreover, we did not investigate patients with lymph node metastases because stage I EAC patients are free from nodular involvement by definition. Currently, the clinical stage and pathological grade of EAC seem to be sufficient predictors in patients with stage I tumours. Long-term follow-up and a larger study population will help validate our results.
In conclusion, the present investigation of early-stage EAC revealed several new observations. First, we described a positive correlation between SOX-2 with age and elevated Oct4 expression in hypertensive patients. Second, the expression of SOX-2 and Oct4 was coordinated only in low-grade EAC. Third, we observed SOX-2, but not Oct4, overexpression in high-grade EAC. Our study suggests that there is no need to use SOX-2 expression as a poor outcome predictor in stage I EAC. However, further investigation of its expression in more advanced stages (II-IV) is justified, particularly in combination with clinicopathological features and known prognostic markers.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Zhou X, Zhou YP, Huang GR, Gong BL, Yang B, Zhang DX, Hu P, Xu SR. Expression of the stem cell marker Nanog in human endometrial adenocarcinoma. Int J Gynecol Pathol. 2011;30:262–270. doi: 10.1097/PGP.0b013e3182055a1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei X, Wang Y, Jing J. Embryonic stem cell markers Sox-2 and OCT4 expression and their correlation with WNT signal pathway in cervical squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:2470–2476. [PMC free article] [PubMed] [Google Scholar]
- 5.Ebben JD, Treisman DM, Zorniak M, Kutty RG, Clark PA, Kuo JS. The cancer stem cell paradigm: a new understanding of tumor development and treatment. Expert Opin Ther Targets. 2010;14:621–632. doi: 10.1517/14712598.2010.485186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubbard S, Gargett C. A cancer stem cell origin for human endometrial carcinoma? Reproduction. 2010;140:23–32. doi: 10.1530/REP-09-0411. [DOI] [PubMed] [Google Scholar]
- 7.Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–646. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Wegner M. All purpose Sox: The many roles of Sox proteins in gene expression. Int J Biochem Cell Biol. 2010;42:381–390. doi: 10.1016/j.biocel.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herreros-Villanueva M, Zhang JS, Koenig A, Abel EV, Smyrk TC, Bamlet WR, de Narvajas AA, Gomez TS, Simeone DM, Bujanda L, Billadeau DD. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis. 2013;2:e61. doi: 10.1038/oncsis.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzino A. The Sox2-Oct4 Connection: Critical Players in a Much Larger Interdependent Network Integrated at Multiple Levels. Stem Cells. 2013;31:1033–9. doi: 10.1002/stem.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Wang YD, Cai N, Wu XL, Cao HZ, Xie LL, Zheng PS. OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death and Disease. 2013;4:e760. doi: 10.1038/cddis.2013.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybicka A, Mucha J, Majchrzak K, Taciak B, Hellmen E, Motyl T, Krol M. Analysis of microRNA expression in canine mammary cancer stem-like cells indicates epigenetic regulation of transforming growth factor-beta signaling. J Physiol Pharmacol. 2015;66:29–37. [PubMed] [Google Scholar]
- 17.Clarke MF, Fuller M. Stem cells and cancer. Two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Lin CW, Liao MY, Lin WW, Wang YP, Lu TY, Wu HC. Epithelial cell adhesion molecule regulates tumor initiation and tumorigenesis via activating reprogramming factors and epithelial-mesenchymal transition gene expression in colon cancer. J Biol Chem. 2012;287:39449–39459. doi: 10.1074/jbc.M112.386235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, Klein B. Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun. 2009;383:157–162. doi: 10.1016/j.bbrc.2009.02.156. [DOI] [PubMed] [Google Scholar]
- 20.Skidan I, Steiniger SC. In vivo models for cancer stem cell research: a practical guide for frequently used animal models and available biomarkers. J Physiol Pharmacol. 2014;65:157–169. [PubMed] [Google Scholar]
- 21.Ruan J, Wei B, Xu Z, Yang S, Zhou Y, Yu M, Liang J, Jin K, Huang X, Lu P, Cheng H. Predictive value of Sox2 expression in transurethral resection specimens in patients with T1 bladder cancer. Med Oncol. 2013;30:445. doi: 10.1007/s12032-012-0445-z. [DOI] [PubMed] [Google Scholar]
- 22.Ji J, Wei X, Wang Y. Embryonic stem cell markers Sox-2 and OCT4 expression and their correlation with WNT signal pathway in cervical squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:2470–2476. [PMC free article] [PubMed] [Google Scholar]
- 23.Yang F, Gao Y, Geng J, Qu D, Han Q, Qi J, Chen G. Elevated expression of SOX2 and FGFR1 in correlation with poor prognosis in patients with small cell lung cancer. Int J Clin Exp Pathol. 2013;6:2846–2854. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, He W, Lu C, Wang Z, Wang J, Giercksky KE, Nesland JM, Suo Z. Oct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human oesophageal squamous cell carcinoma. Anticancer Res. 2009;29:1233–1241. [PubMed] [Google Scholar]
- 25.Abd EI-Maqsoud NM, Adb EI-Rehim DM. Clinicopathologic implications of EpCAM and Sox2 expression in breast cancer. Clin Breast Cancer. 2013;27:S1526–8209. doi: 10.1016/j.clbc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, Miki C, Kusunoki M. Correlation of CD133, Oct4 and Sox2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16:3488–3988. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]
- 27.Abd El-Maqsoud NM, Abd El-Rehim DM. Clinicopathologic implications of EpCAM and Sox2 expression in breast cancer. Clin Breast Cancer. 2014;14:e1–9. doi: 10.1016/j.clbc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, Miki C, Kusunoki M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16:3488–98. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]