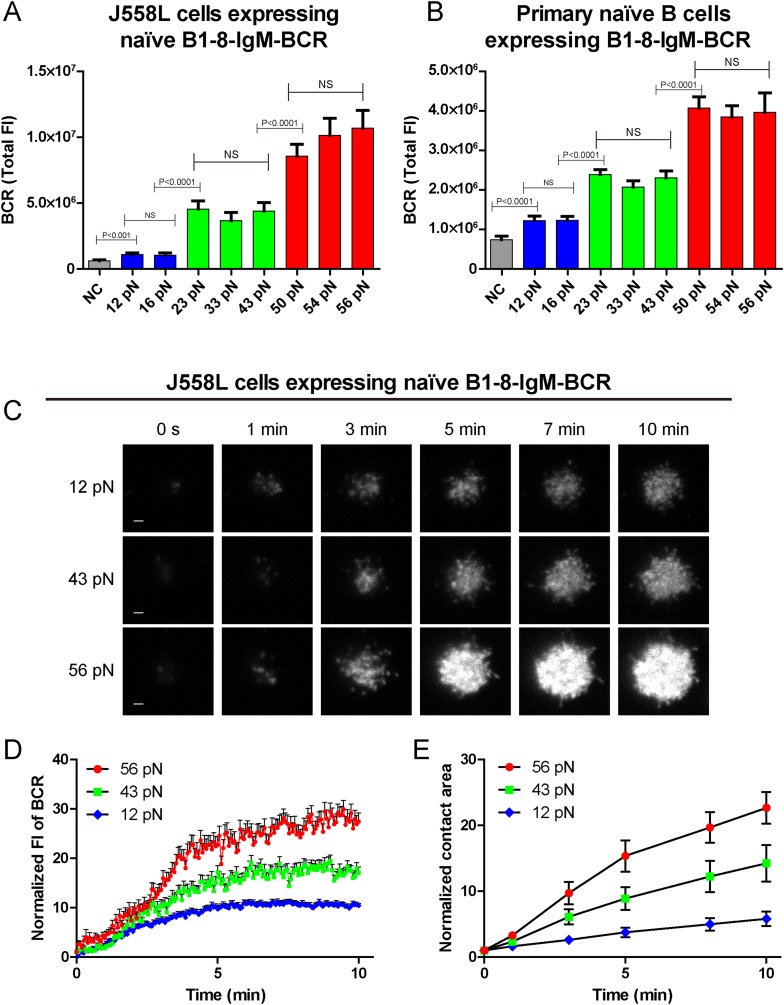
Figure 2. The synaptic accumulation of the IgM-BCRs is dependent on mechanical forces and exhibits a multi-threshold effect.
(A, B) Statistical quantification of the synaptic recruitment of IgM-BCR in J558L cells expressing naive B1-8-IgM-BCR (A) and primary naive B cells expressing B1-8-IgM-BCR (B). Bars represent mean ±SEM. Two-tailed t tests were performed for the statistical comparisons. Data are from at least 40 cells over three independent experiments. (C) Representative TIRFM images showing the dynamics of the synaptic accumulation of IgM-BCRs from J558L cells expressing naive B1-8-IgM-BCR in contact with coverslip presenting 12 pN, 43 pN or 56 pN NP-TGT sensors at the indicated time points. Scale bar is 1.5 μm. (D, E) Comparisons of averaged traces showing the dynamic accumulation of naive IgM-BCRs into the immunological synapse (D) and the growing features of the size of contact area (E) for J558L cells expressing naive B1-8-IgM-BCR as demonstrated in (C) in a 10 min TIRFM imaging time course. Bars represent mean ±SEM. Two-tailed t tests were performed for the statistical comparisons. Data are from at least 20 cells over two independent experiments.