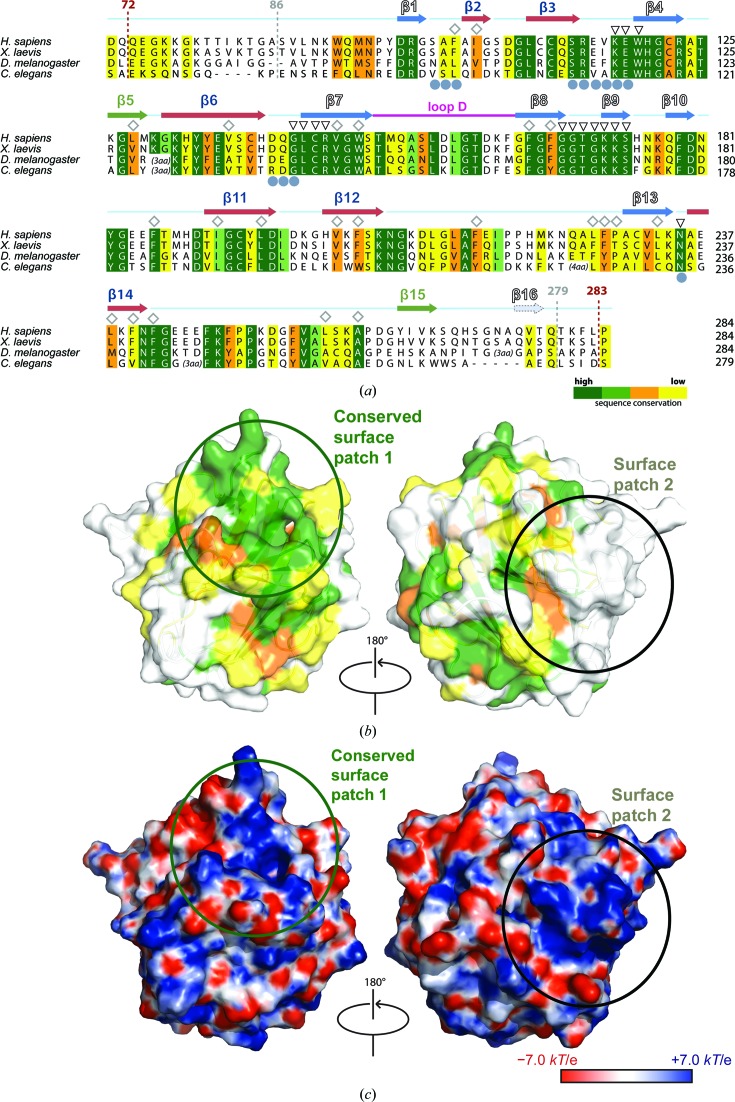
Figure 5.
Sequence conservation of the SPRY domain amongst DDX1 homologues. (a) Sequence alignment of hDSPRY with the SPRY domains of DDX1 homologues from eukaryotic model organisms. Conservation values were determined using the AMAS server (Livingstone & Barton, 1993 ▸) and are indicated by colour coding (dark green for identical residues to yellow for less homologous residues). Residues of the hydrophobic core that stabilize the domain fold are indicated by diamonds, residues of surface A are indicated by grey circles and residues of the conserved, positively charged surface patch of hDSPRY are indicated by triangles. Secondary-structure elements are shown above the sequence alignment and are coloured according to Fig. 1 ▸. Residues that could be modelled in the crystal structure (residues 86–279) are indicated in grey and domain boundaries of the crystallization construct are indicated in brown (residues 72–283). (b) Sequence conservation mapped onto the molecular surface of hDSPRY. (c) Electrostatic surface potential, calculated using APBS (Baker et al., 2001 ▸), mapped onto the molecular surface.