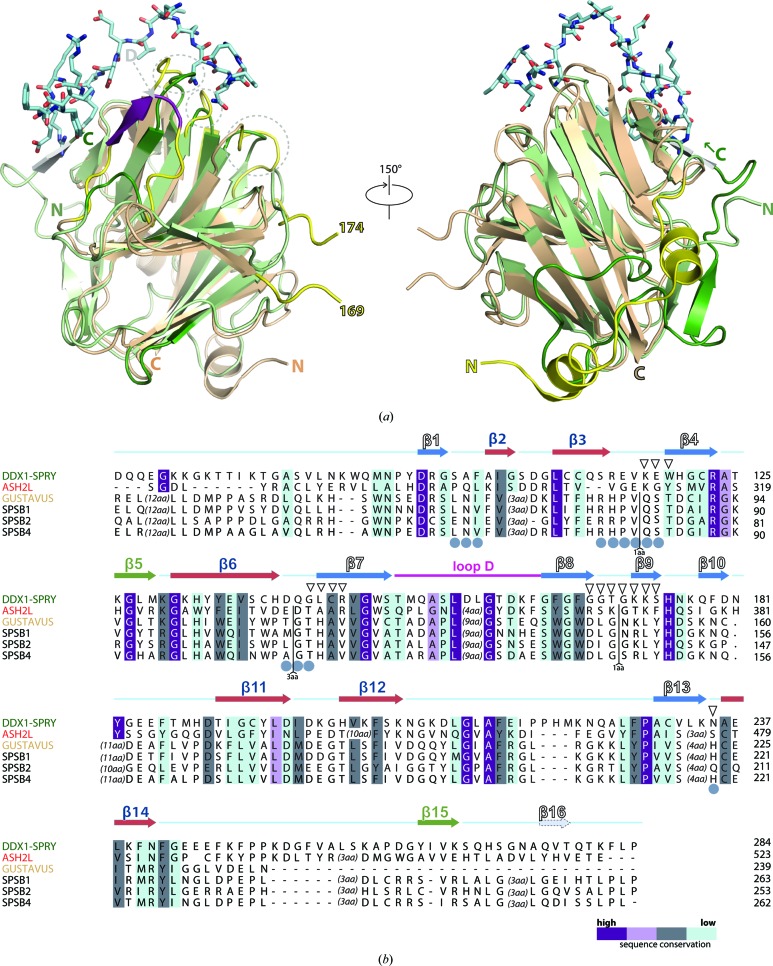
Figure 6.
Comparison of the interaction surface of SPRY complex structures. (a) DALI superposition of hDSPRY (green) with the SPRY domain of D. melanogaster GUSTAVUS (yellow) and a 20-residue VASA peptide (shown as a stick model; PDB entry 2ihs; Woo, Suh et al., 2006 ▸). Loop D is marked in purple. The loop region between residues 169 and 174 in GUSTAVUS is not resolved. (b) Sequence alignment of hDSPRY with the best hits from the DALI search. Conservation values were determined using the AMAS server (Livingstone & Barton, 1993 ▸) and are indicated by colour coding (from dark purple for identical residues to light blue for less homologous residues). Residues of surface A of related SPRY domains are labelled with grey circles, and residues of the conserved, positively charged surface patch of hDSPRY are labelled with triangles.