Abstract
Aims:
The aim was to correlate the incidence of metastasis to Level IIB of neck lymph nodes (LNs) for oral cavity carcinomas with the site, size, and histological grade of tumor.
Settings and Design:
Total 30 patients of either sex, with biopsy-proven oral squamous cell carcinoma of any site, size or histologic grade, but N0/N1 were taken for selective neck dissection (SND).
Materials and Methods:
Thirty patients who underwent SND for oral carcinoma were analyzed for the relation of the site, size, and histological grade of malignancy with metastatic involvement to Level IIB nodes. Level IIB nodes were dissected separately and sent for histopathological examination.
Statistical Analysis Used:
The data were entered in custom written software in Excel (MS office 2007, Windows XP) and the data were analyzed using statistical software STATA version 10.0. The statistical test used for the analysis of the result was Chi-square test. The critical level of statistical significance chosen was P < 0.05.
Results:
Only 2 of 30 patients (6.6%) had the involvement of Level IIB neck nodes. There was no relation between the site, size, and histologic grade of primary tumor with the metastasis to Level IIB. The Level IIA nodes were positive in both the positive cases of Level IIB.
Conclusions:
For tumors in oral cavity (N0/N1), while performing elective or therapeutic SND the dissection of Level IIB nodes could be omitted as it will provide significant decrease in operative time and also less of spinal accessory nerve trauma-related complications.
Keywords: Level IIB nodes, neck dissection, oral cancer, site, and size of tumor
INTRODUCTION
The most important prognostic factor in the management of squamous cell carcinoma (SCC) of the oral cavity is still the presence of cervical nodal metastasis, as a century ago. Once the tumor involves neck nodes, survival drops by almost 50%.[1] Improvements in surgical modalities and functional results over the last 10 decades have been based on technical and on philosophical considerations.
In spite of advancement in science, molecular medicine and target therapies, surgical treatment of metastasis using different techniques, from selective neck dissection (SND) to extended radical neck dissections, form a major part in the management of neck metastasis. This is due to the fact that, so far, there is no treatment more effective for resectable neck metastasis, than surgery.
Kocher in 1880 was the first person to present a conceptual approach for removing nodal metastasis.[2] George in 1906, presented a series of 132 neck dissections, and described the classic technique of the radical neck dissection.[3] Originally, this technique included removal of the submandibular salivary gland, internal jugular vein, greater auricular and spinal accessory nerves (SANs), as well as the digastric, stylohyoid, and sternocleidomastoid muscles.
Suen and Goepfert further subdivide areas of differing lymphatic drainage within certain levels.[4]
The subzones IA, IB, IIA, IIB, IVA, IVB, VA, and VB which were not part of the original description of the levels of the neck were thus included. Increased knowledge of the regional spread of tumors and a desire to minimize operative morbidity have led to the widespread use of SND as a staging or therapeutic procedure in the management of cancer patients.[5,6]
This has enabled us to adopt modified and SNDs which have ultimately led to a dramatic reduction in morbidity and almost eliminated mortality due to neck dissection.[7] Depending on the site of the primary tumor, the subzones may have biological significance and can guide decision-making in determining which nodal levels should be addressed surgically. By removing only those nodal groups considered high risk for metastasis based on the primary tumor site and by preserving key non-lymphatic structures, SND retains the oncological effectiveness of the radical neck dissection but avoids much of the associated morbidity.
Patient survival and regional control following SND are comparable to those of more extensive neck dissections in the clinically N0 or in some instances, the node-positive neck.[8]
One of the more technically difficult aspects of SND is a dissection of the upper jugular and spinal accessory LNs in the posterior region of Level II. This area has been previously referred to as Level IIB,[4] the supraretrospinal triangle, the supraspinal accessory LN pad, and more recently, the submuscular recess (SMR).[9]
The concept of sublevels is clinically relevant since LN metastasis to Level IIB are quite rare.[10] Limiting unnecessary dissection of nodal sublevels unlikely to harbor metastatic disease results in greater preservation of function of important clinical structures in the neck, particularly the SAN. Minimal dissection and skeletonization of the SAN provides the best functional results.
This study is an analysis along with systematic review of literature in the same direction, involving a series of consecutive patients undergoing neck dissection to further characterize the prevalence of nodal metastasis in the SMR or Level IIB in Indian population.
Metastatic involvement of the Level IIB as it relates to the primary tumor site, tumor size, and histologic grade of malignancy are also discussed.
MATERIALS AND METHODS
A total of 30 cases with histopathologically proven oral SCC (OSCC) classified according to AJCC 2005 (sixth edition) with preoperative neck status (N0 or N1) were undertaken for the study. All the procedures were carried out under general anesthesia wherein the patients were intubated using the nasotracheal intubation.
The neck was dissected first and the nodes from Level I-III (Therapeutic and elective, Nodes dependent) were resected en bloc along with the primary tumor inside the oral cavity trying to keep at least 2 cm clear margins all around. Level IIB was dissected exclusively and nodes were excised, labeled, and later sent for histological examination separately from the rest of the specimen.
The study included 30 patients which were in the age group of 22–70 years. The mean age was 50.50 years. There were 22 male patients and 8 female patients.
The distribution of patients according to primary site and size of tumor was according to site, 2 patients had tumor at anterior alveolus mandible, 2 patients had tumor at gingivobuccal sulcus, 5 patients had tumor at tongue, 9 patients had tumor at posterior alveolus mandible, 8 patients had tumor at buccal mucosa, and 4 patients had tumor at retromolar trigone.
According to the size of primary tumor, 4 patients had primary of size T1, 14 had primary of size T2, 4 had primary of size T3, and 8 had primary of size T4a.
Mean number of LNs harvested from each level were-Level IA had 3.13 (standard deviation [SD] ± 2.19), Level IB had 3.20 (SD ± 1.51), Level IIA had 3.63 (SD ± 2.23), Level IIB had 4.06 (SD ± 2.13), and Level III had 4.06 (SD ± 2.24).
RESULTS
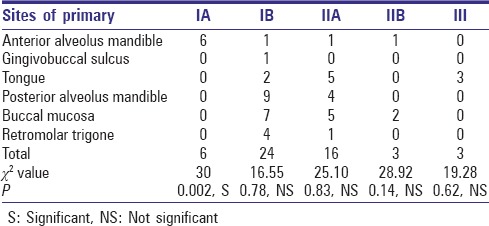
The site of tumor when co-related with positive nodes at different levels in neck for their role in influencing the metastasis pattern was non-significant except for tumors at anterior alveolus of mandible which had significant co-relation with the pattern of drainage to Level IA [Table 1].
Table 1.
Number of positive nodes according to the site of primary tumor
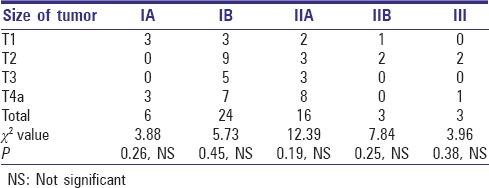
The size of primary tumor when co-related with positive nodes at different levels in the neck for their role in influencing the metastasis pattern was again non-significant on statistical analysis for any particular tumor size [Table 2].
Table 2.
Number of positive nodes according to the size of the primary tumor
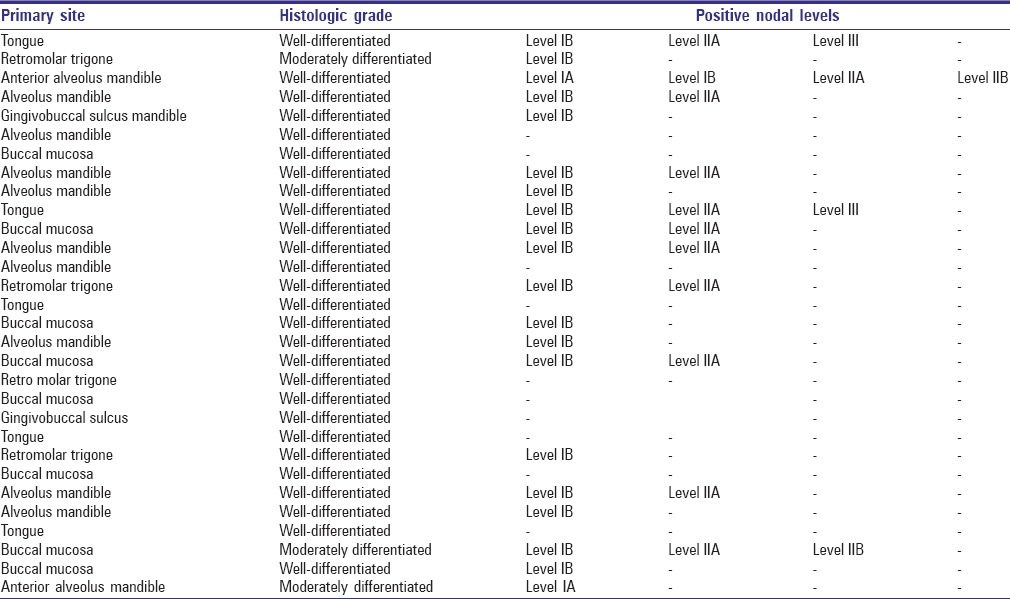
While co-relating the histologic grade of malignancy and pattern of metastasis to a different level of nodes in neck, all the patients showed metastasis limited to Level I and II. Level III was involved only in 2 cases which were well-differentiated OSCC of tongue in both the cases. All the patients had histologic Grade I or II of malignancy [Table 3].
Table 3.
Co-relation of histologic grade with positive levels
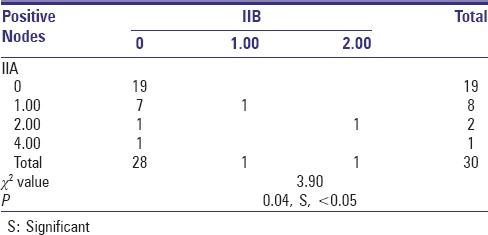
The most significant result of the study was found while co-relating the incidences of metastasis to Level IIA with Level IIB. On statistical evaluation, it was suggested that there was no incidence of isolated metastasis to Level IIB without the evidence of metastasis to Level IIA (P < 0.05) [Table 4].
Table 4.
Co-relation of IIB with IIA (positive nodes) in patients
DISCUSSION
Squamous cell carcinoma of the oral cavity accounts for 4% of all malignancies in men and 2% of all malignancies in women, and constitutes almost 3% of all cancer deaths.[11] A cervical LN metastasis is one of the most significant prognostic factors in patients with SCC of the oral cavity. The surgical options for managing neck metastasis include a classic radical neck dissection, a modified radical neck dissection, and a SND. Shah[6] reported that regional metastasis of SCC of the oral cavity was generally located in Levels I, II, and III. In addition, they reported that the risk of a Level IV or V LN metastasis was extremely low. Therefore, supra omohyoid neck dissection (SOHND) is becoming increasingly popular and acceptable for elective treatment in managing clinically N0 necks in patients with SCC of the oral cavity.
In the present study, the mandibular alveolus and buccal mucosa were the most frequently involved sites (30% and 26.66%, respectively), while the anterior mandibular alveolus and gingivobuccal sulcus were the least commonly involved sites (6.6% each). These regional differences may be attributed to the exclusive use of chewing tobacco in the Indian subcontinent compared to smoking in the West.[12]
“The size of the lesion may reflect the social conditions of the population and their ability to obtain healthcare.”[13] India has a great social and economic discrepancy, and most patients who seek health care in the public hospitals consist of people from a weaker and economically poorer section of society. In our study, we observed mostly lesions with a size T2 and T4a (46.7% and 26.7%, respectively) and less frequently of size T1 and T3 (13.3% each) which can be compared to the work of Luciana S. Marocchio et al., and Oji and Chukwuneke.[13] Recently, the general indications for performing SOHND for SCC of the oral cavity have been extended to therapeutic lymphadenectomy in conjunction with postoperative radiotherapy for a minimal nodal metastasis confined to the first echelon of the lymphatic drainage (N1) as well an elective lymphadenectomy in patients with clinically negative nodal disease (N0) at high risk for cervical metastasis.
There may be various postoperative morbidities after a SOHND, and one of those is postoperative shoulder dysfunction, which occurs less but frequently, as compared to radical neck dissection.
Shoulder syndrome because of radical neck dissection was first described by Nahum et al.[14] Findings of this syndrome are shoulder pain, restricted abduction, a normal passive range of motion, pathoanatomical changes (shoulder drop, muscle atrophy, wing scapula), and abnormal electroneuromyographic changes. Shoulder syndrome is considered to be a result of SAN injury.[15,16] The area in neck, which during dissection is most likely to cause damage to SAN, is Level IIB. The boundaries of Level II extend from the level of the skull base superiorly to the level of the lower border of the hyoid bone inferiorly. The anterior (medial) boundary of Level II is the posterior belly of digastric/stylohyoid muscle and the posterior (lateral) boundary is the posterior border of the sternocleidomastoid muscle. The levator scapula and splenius capitis muscles form the bed of this anatomical area[4,17].
Level II LNs are located around the upper third of the internal jugular vein and have a close relationship with the SAN. It crosses surgical neck Level II obliquely in a superoinferior and mediolateral direction dividing it into two parts, the posterosuperolateral part of which has been termed sublevel IIB[4].
The latest classification of neck dissection by the American Head and Neck Society and the American Academy of Otolaryngology – Head and Neck Surgery recommended dividing Level II into sublevels A and B.[17] The nodes within sublevel IIA are located anteriorly to the vertical plane defined by the SAN, while those in sublevel IIB are located posterior to this plane. Sublevel IIB has been reported to contain a median of 4.2 nodes per specimen.
Similarly in our study, we found the mean number of nodes harvested from Level IIB was 4.06. A clear association between the extent of the dissection and the number of harvested LNs was observed.
Total number of LNs in a specimen appears to be strongly dependent on the harvesting protocol and the extent of the dissection. The mean number of LNs among 30 specimens examined by pathologists in our study was 26.06 (range 13–42) which was comparable to the study by Marres et al.,[18] in which they suggested the mean of total LNs in neck dissection specimen to be 24 with range 0–89 and also with the study of Bhattacharyya,[19] who concluded that the modified radical neck dissection has the maximum yield of nodes (26.3).
“The quality of life after neck dissection is significantly improved if the function is preserved. Morbidity can be prevented by SND in selected cases.”[17] It has recently been reported that the probability of metastasis in Level IIB is very low.[20]
Technical descriptions reported by a number of other studies stressed the importance of including Level IIB LNs during SOHND.[5]
In our study, we co-related the incidence of metastasis to various levels of neck with the site and size (T) of primary tumor in the oral cavity.
When co-relating with the site of primary tumor, we found that only primary tumors of anterior alveolus of mandible have significant correlation with the drainage pattern to Level IA (P = 0.002) [Figure 1].
Figure 1.
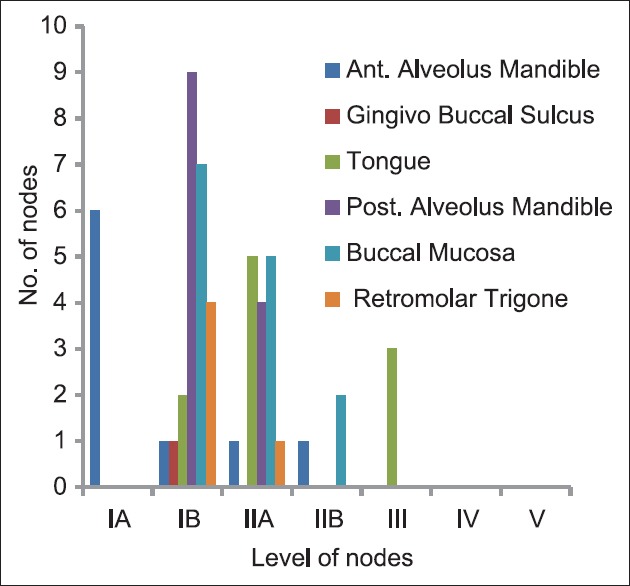
Number of positive nodes according to the site of primary tumor
When co-relating with size of primary tumor, we found that size of primary tumor does not affect the pattern of drainage to any specific level (P > 0.05), which was comparable to the studies by Umeda et al.,[21] and Akhter et al.,[22] who stated that the prevalence of neck metastasis was not significantly co-related with the primary site and T stage [Figure 2].
Figure 2.
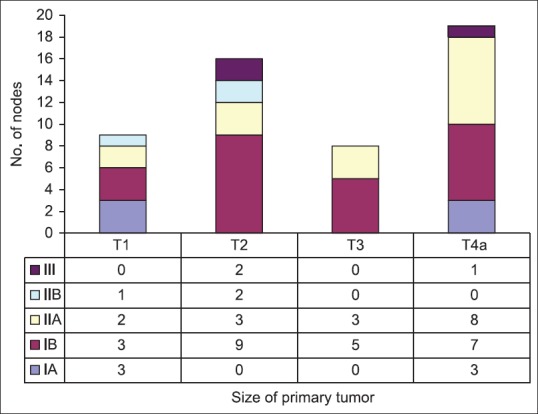
Number of positive nodes according to the size of the primary tumor
The risk of nodal disease in Level IIB is greater for tumors arising in the oropharynx compared with the oral cavity and larynx. Thus, in the absence of clinical nodal disease in Level IIA, it is likely not necessary to include Level IIB for tumors arising in these latter sites. It is probable that leaving Level IIB undissected will result in a minimal deteriorative effect on the SAN as well as decrease the operative time.
In our study, the maximum number of patients had histopathologically proven positive nodes at Level IB (63.33%), followed by Level IIA (36.67%), Level IA (6.67%), Level IIB (6.67%), and Level III (6.67%) which was in conjunction with studies of Chone et al.,[20] who stated that metastasis to Level IIB is 6.5%, Smith et al.,[23] stated it to be 8.75%, Villaret et al.,[24] stated it to be 5.67%, Elsheikh et al.,[25] gave an incidence of 7.3%, and Lea et al.,[26] found that the incidence of metastasis to Level IIB is 6.0% [Figure 3].
Figure 3.
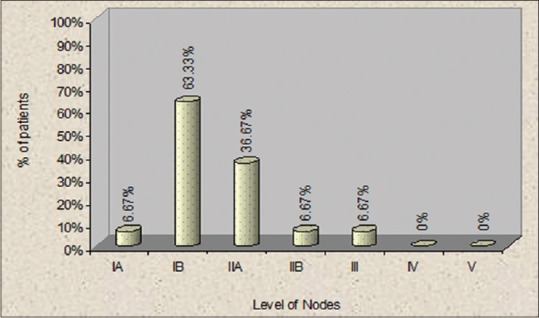
Percentage of patients with positive nodes at different levels of neck
None of the patients showed metastasis to Level IV and Level V during follow-up and imaging studies which were in conjunction with the findings of Shah.[6]
We found that the most common involved site in the neck was Level IB followed by Level IIA, which was comparable to study done by Pugazhendi et al.,[27] who also concluded with the same results as ours. The studies not in conjunction with our results were the studies by Tao et al.,[28] and Vartanian et al.,[29] who stated that Level IIA is the most commonly involved site in metastasis.
Furthermore, in both the patients with positive Level IIB nodes there were always positive nodes in Level IIA. There was a significant correlation between the incidence of metastasis to Level IIB and Level IIA (P < 0.05), which means that the metastasis to Level IIB is always associated with Level IIA and never independent of it which was comparable to studies by Lea et al.,[26] Elsheikh et al.,[30] and Chone et al.[20] All of them suggested that there are no incidences of isolated metastasis to Level IIB and if metastasis to Level IIB is there then it is always in conjunction with Level IIA [Figure 4].
Figure 4.
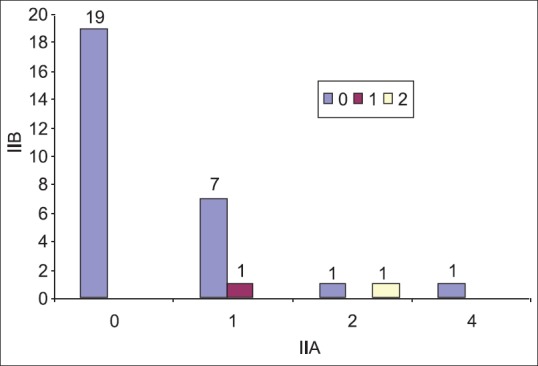
Co-relation of IIB with IIA (positive nodes) in patients
In the present study, we evaluated the histological incidence of neck LNs at various levels and co-related it with size, site, and histologic grade of malignancy of primary tumor in OSCC giving special emphasis to Level IIB nodes.
We were able to efficiently co-relate the said parameters and found no significant relation of site and size of tumor to the nodal drainage except for tumors at anterior mandibular alveolus which showed definite pattern of metastasis to Level IA.
We also found a definite co-relation between the histologic grade of malignancy and the extent of neck nodal metastasis, which was comparable to the studies done by Umeda et al.,[21] and Akhter et al.[22] According to them patients with Grade I-II histologic malignancy showed limited metastases that involved LNs in Levels I-II. On the other hand, patients showing Grade III-IV histologic malignancy often had metastases that extended beyond Level III, regardless of T stage.
A statistically significant result was achieved suggesting that the incidence of metastasis to Level IIB is very rare and is significantly associated with metastasis to Level IIA.
CONCLUSION
We would suggest that the type of neck dissection to be considered while dissecting for primaries of the oral cavity with histologic Grade I and II of malignancy should be SND (I-III).
Exploration of Level IIB is not mandatory in all cases, but should be undertaken whenever there is extensive involvement of Level IIA clinically which will greatly influence the postoperative morbidity of neck and shoulder.
As it was very difficult to find any study in published English literature pertaining to Level IIB metastasis in neck and oral carcinomas conducted in India, it was our sincere effort, though in a small sample size to bring out an issue which lacks in research from this particular part of world in spite of having the maximum number of patients with oral carcinomas.
Further, more prospective and multi-institutional studies are required especially pertaining to exclusive cases of oral malignancies and their biological behavior.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chiesa F. Centenary of Crile's operation. From radical to selective neck dissection. Acta Otorhinolaryngol Ital. 2006;26:307–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Kocher T. About radical cure of cancer. Dtsch Z Chir. 1880;13:134–66. [Google Scholar]
- 3.George C. Excision of cancer of the head and neck. J Am Med Assoc. 1906;47:1780–6. [Google Scholar]
- 4.Suen JY, Goepfert H. Standardization of neck dissection nomenclature. Head Neck Surg. 1987;10:75–7. doi: 10.1002/hed.2890100202. [DOI] [PubMed] [Google Scholar]
- 5.Bocca E, Pignataro O, Oldini C, Cappa C. Functional neck dissection: An evaluation and review of 843 cases. Laryngoscope. 1984;94:942–5. doi: 10.1288/00005537-198407000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405–9. doi: 10.1016/s0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 7.Subramanian S, Chiesa F, Lyubaev V, Aidarbekova A. The evolution of surgery in the management of neck metastases. Acta Otorhinolaryngol Ital. 2006;26:309–16. [PMC free article] [PubMed] [Google Scholar]
- 8.Medina JE, Byers RM. Supraomohyoid neck dissection: Rationale, indications, and surgical technique. Head Neck. 1989;11:111–22. doi: 10.1002/hed.2880110203. [DOI] [PubMed] [Google Scholar]
- 9.Calearo CV, Teatini G. Functional neck dissection. Anatomical grounds, surgical technique, clinical observations. Ann Otol Rhinol Laryngol. 1983;92:215–22. doi: 10.1177/000348948309200301. [DOI] [PubMed] [Google Scholar]
- 10.Rinaldo A, Robbins KT, Ferlito A. The importance of distinguishing between sublevel IA and IB in neck dissection. ORL J Otorhinolaryngol Relat Spec. 2004;66:53–5. doi: 10.1159/000077794. [DOI] [PubMed] [Google Scholar]
- 11.Silverberg E. Cancer statistics 1986. CA Cancer J Clin. 1986;36:9–25. doi: 10.3322/canjclin.36.1.9. [DOI] [PubMed] [Google Scholar]
- 12.Gorsky M, Epstein JB, Oakley C, Le ND, Hay J, Stevenson-Moore P. Carcinoma of the tongue: A case series analysis of clinical presentation, risk factors, staging, and outcome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:546–52. doi: 10.1016/j.tripleo.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 13.Oji C, Chukwuneke FN. Oral cancer in Enugu, Nigeria, 1998-2003. Br J Oral Maxillofac Surg. 2007;45:298–301. doi: 10.1016/j.bjoms.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Nahum AM, Mullally W, Marmor L. A syndrome resulting from radical neck dissection. Arch Otolaryngol. 1961;74:424–8. doi: 10.1001/archotol.1961.00740030433011. [DOI] [PubMed] [Google Scholar]
- 15.Kiroglu M, Sarpel T, Özberk P, Soylu L, Çetik F, Özsahinoglu C. Signs of shoulder syndrome in neck dissections. Turk Arch Otolaryngol. 1995;33:254–9. [Google Scholar]
- 16.Gordon SL, Graham WP, rd, Black JT, Miller SH. Acessory nerve function after surgical procedures in the posterior triangle. Arch Surg. 1977;112:264–8. doi: 10.1001/archsurg.1977.01370030036005. [DOI] [PubMed] [Google Scholar]
- 17.Robbins KT, Clayman G, Levine PA, Medina J, Sessions R, Shaha A, et al. Neck dissection classification update: Revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128:751–8. doi: 10.1001/archotol.128.7.751. [DOI] [PubMed] [Google Scholar]
- 18.Marres CC, de Ridder M, Hegger I, van Velthuysen ML, Hauptmann M, Navran A, et al. The influence of nodal yield in neck dissections on lymph node ratio in head and neck cancer. Oral Oncol. 2014;50:59–64. doi: 10.1016/j.oraloncology.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharyya N. The effects of more conservative neck dissections and radiotherapy on nodal yields from the neck. Arch Otolaryngol Head Neck Surg. 1998;124:412–6. doi: 10.1001/archotol.124.4.412. [DOI] [PubMed] [Google Scholar]
- 20.Chone CT, Crespo AN, Rezende AS, Carvalho DS, Altemani A. Neck lymph node metastases to the posterior triangle apex: Evaluation of clinical and histopathological risk factors. Head Neck. 2000;22:564–71. doi: 10.1002/1097-0347(200009)22:6<564::aid-hed4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Umeda M, Yokoo S, Take Y, Omori A, Nakanishi K, Shimada K. Lymph node metastasis in squamous cell carcinoma of the oral cavity: Correlation between histologic features and the prevalence of metastasis. Head Neck. 1992;14:263–72. doi: 10.1002/hed.2880140402. [DOI] [PubMed] [Google Scholar]
- 22.Akhter M, Hossain S, Rahman QB, Molla MR. A study on histological grading of oral squamous cell carcinoma and its co-relationship with regional metastasis. J Oral Maxillofac Pathol. 2011;15:168–76. doi: 10.4103/0973-029X.84485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith R, Taylor SM, Trites JR, Smith A. Patterns of lymph node metastases to the submuscular recess. J Otolaryngol. 2007;36:203–7. doi: 10.2310/7070.2007.0033. [DOI] [PubMed] [Google Scholar]
- 24.Villaret AB, Piazza C, Peretti G, Calabrese L, Ansarin M, Chiesa F, et al. Multicentric prospective study on the prevalence of sublevel IIb metastases in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:897–903. doi: 10.1001/archotol.133.9.897. [DOI] [PubMed] [Google Scholar]
- 25.Elsheikh MN, Rinaldo A, Ferlito A, Fagan JJ, Suárez C, Lowry J, et al. Elective supraomohyoid neck dissection for oral cavity squamous cell carcinoma: Is dissection of sublevel IIB necessary? Oral Oncol. 2008;44:216–9. doi: 10.1016/j.oraloncology.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Lea J, Bachar G, Sawka AM, Lakra DC, Gilbert RW, Irish JC, et al. Metastases to level IIb in squamous cell carcinoma of the oral cavity: A systematic review and meta-analysis. Head Neck. 2010;32:184–90. doi: 10.1002/hed.21163. [DOI] [PubMed] [Google Scholar]
- 27.Pugazhendi SK, Thangaswamy V, Venkatasetty A, Thambiah L. The functional neck dissection for lymph node neck metastasis in oral carcinoma. J Pharm Bioallied Sci. 2012;4:S245–7. doi: 10.4103/0975-7406.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao L, Lefèvre M, Callard P, Périé S, Bernaudin JF, St Guily JL. Reappraisal of metastatic lymph node topography in head and neck squamous cell carcinomas. Otolaryngol Head Neck Surg. 2006;135:445–50. doi: 10.1016/j.otohns.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Vartanian JG, Pontes E, Agra IM, Campos OD, Gonçalves-Filho J, Carvalho AL, et al. Distribution of metastatic lymph nodes in oropharyngeal carcinoma and its implications for the elective treatment of the neck. Arch Otolaryngol Head Neck Surg. 2003;129:729–32. doi: 10.1001/archotol.129.7.729. [DOI] [PubMed] [Google Scholar]
- 30.Elsheikh MN, Mahfouz ME, Elsheikh E. Level IIb lymph nodes metastasis in elective supraomohyoid neck dissection for oral cavity squamous cell carcinoma: A molecular-based study. Laryngoscope. 2005;115:1636–40. doi: 10.1097/01.mlg.0000176540.33486.c3. [DOI] [PubMed] [Google Scholar]


