Abstract
Objective
This study aims to investigate the potential benefit of combination therapy with magnetic nanoparticles of Fe3O4 (Fe3O4-MNP) and gambogic acid (GA) on SMMC-7721 cells.
Methods
The inhibition of proliferation of SMMC-7721 cells was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Cell apoptosis was calculated and analyzed by flow cytometry, and the expressions of the apoptosis-related protein were detected by Western blot.
Results
GA enhanced the cytotoxicity of SMMC-7721 cells in a dose-dependent manner. The Fe3O4-MNP itself had no obviously inhibitory effect, but it could enhance the effect of GA on proliferation of SMMC-7721 cells. The apoptotic rate of SMMC-7721 cells induced by combination of GA with Fe3O4-MNP was higher than that by GA alone. The expression levels of caspase-3 and caspase-8 after co-treatment of GA and Fe3O4-MNP were higher than that exposed to either GA or Fe3O4-MNP alone, while the levels of bcl-2 were downregulated.
Conclusion
Fe3O4-MNP can promote GA-induced apoptosis of SMMC-7721 cells, which may be related to the downregulation of Bcl-2 and upregulation of caspase-3.
Keywords: primary hepatocellular carcinoma, traditional Chinese medicine, anti-tumor activity, targeted-drug carrier
Introduction
Gambogic acid (GA; C38H44O8) (Figure 1) is one of active components of gamboge, a traditional Chinese medicine, which is a dry resin secreted from Garcinia hanburyi.1 It is reported in traditional Chinese medicine that GA is cold, acidic, acerbic, and poisonous. In addition, GA has a significant anticancer effect on a wide variety of solid tumors, including lymphoma, colorectal cancer, and glioblastoma.2–4 However, the molecular mechanisms of its antitumor activity and its effects on hepatocellular carcinoma (HCC) cells are still poorly understood and await further investigations.5
Figure 1.
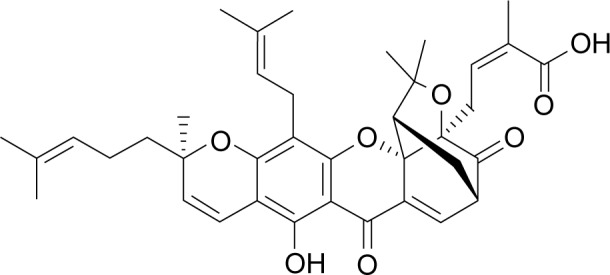
Molecular structure of GA.
Abbreviation: GA, gambogic acid.
HCC is the most common type of liver cancer. It is the third leading cause of cancer-related death globally. Most cases of HCC are secondary to either a viral hepatitis infection (hepatitis B or C) or cirrhosis. The prognosis of patients with HCC remains poor despite the recent therapeutic advances in HCC treatment. Therefore, the identification of the new biomarkers for HCC will supply an arm for improving diagnosis and management of human HCC.
Magnetic nanoparticles of Fe3O4 (Fe3O4-MNP), a kind of biocompatible nanomaterial with low toxicity, is widely used as a targeted-drug carrier with target and sustained-release properties.6
Our study aims to evaluate the potential benefit of combination therapy with GA and Fe3O4-MNP for HCC and whether Fe3O4-MNP could promote the GA-induced apoptosis. To elucidate the mechanisms possibly involved, we also measured the expression of apoptosis-related proteins.
Materials and methods
Materials
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). RPMI 1640 medium was from Gibco/BRL (Thermo Fisher Scientific, Waltham, MA, USA). Newborn calf serum was from Hangzhou Sijiqing Biological Engineering Materials Co., Ltd. (Hangzhou, People’s Republic of China). Annexin V–Fluorescein Isothiocyanate Apoptosis Detection Kit and ECL (enhanced chemiluminescence) Western Blotting Detection Kit were from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, People’s Republic of China). Monoclonal antibodies, including caspase-3, caspase-8, survivin, and Bcl-2, were supplied by Santa Cruz Biotechnology Inc. (Dallas, TX, USA).
Gambogic acid
GA (Jiangsu Key Laboratory of Carcinogenesis and Intervention, China Pharmaceutical University, People’s Republic of China) was dissolved in DMSO, stored at −20°C, and then diluted in RPMI 1640 medium.
Preparation of Fe3O4-MNP
Fe3O4-MNP was compounded and characterized by State Key Laboratory of Bioelectronics (Southeast University, People’s Republic of China). The Fe3O4-MNP was synthesized by electrochemical deposition under oxidizing conditions. Transmission electron microscopy indicated that the majority of Fe3O4-MNPs were spherical in shape with particle sizes of approximately 30 nm (Figure 2).7 Before use, the Fe3O4-MNPs were well dispersed in RPMI 1640 medium containing 10% (v/v) heat-inactivated newborn calf serum using ultrasound to obtain a colloidal suspension of Fe3O4-MNP.
Figure 2.

Image and mean size of magnetic nanoparticles of Fe3O4 under transmission electron microscope.
Note: Copyright © 2013. Dove Medical Press. Reproduced from Li K, Chen B, Xu L, et al. Reversal of multidrug resistance by cisplatin-loaded magnetic Fe3O4 nanoparticles in A549/DDP lung cancer cells in vitro and in vivo. Int J Nanomedicine. 2013;8:1867–1877.7
Cell lines and culture conditions
Primary HCC SMMC-7721 cells were constantly preserved in our laboratory, which were cultured in RPMI 1640 medium containing 10% (v/v) heat-inactivated newborn calf serum at 37°C in a fully humidified incubator with 5% CO2.
Safe dose of Fe3O4-MNP
SMMC-7721 cells were cultured with different concentrations of Fe3O4-MNP (0–20 μg/mL) at 37°C for 24 hours, 48 hours, and 72 hours, respectively, and then the proliferation of cells was evaluated by MTT assay.
Inhibition of proliferation by MTT
As shown in our preliminary experiments, the inhibitory effects of the drugs were the most significant for 48 hours. So we chose 48 hours for the following experiments.
SMMC-7721 cells (5×104 cells per well) were incubated in a 96-well flat-bottomed plate (CoStar, Cambridge, MA, USA). Different concentrations of GA and 20 μg/mL Fe3O4-MNP were added separately and cultured at 37°C for 48 hours. A total of 20 μL of MTT solution (5 mg/mL) was added and incubated at 37°C for 4 hours, and then the optical density (OD) value was read at 540 nm using a plate reader. The inhibition rate of cells was determined as follows: (1− OD of treated group/OD of control group) ×100%.
Apoptosis assay by flow cytometry
SMMC-7721 cells (5×105 cells per well) were incubated in a six-well flat-bottomed plate (CoStar). In all cells, 1 μmol/L GA and 20 μg/mL Fe3O4-MNP were added separately and cultured at 37°C for 48 hours. The cells were collected and suspended in 500 μL of binding buffer, and 5 μL of Annexin-V–fluorescein isothiocyanate and 5 μL of propidium iodide were added, followed by incubation at room temperature for 15 minutes in the dark. Analyses were performed by using flow cytometry (BD, Franklin Lakes, NJ, USA).
Western blot analysis
After drug treatment, total protein was isolated from the SMMC-7721 cells (Whole Cell Lysis Assay, KGP250; KeyGen Biotech Co., Ltd.) and measured using BCA protein quantitation assay kit (KGPBCA; KeyGen Biotech Co., Ltd.). Then, it was subjected to SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The blots were stained overnight with primary antibody (1:1,000–1,200) at 4°C and then with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000) for 1 hour at room temperature. The blots were visualized using the ECL system (Amersham, UK). Images were analyzed using ImageJ software from the National Institutes of Health (Bethesda MD, USA). β-Actin was used as an internal standard.
Statistical analysis
All experiments were repeated for at least three times. Data were expressed as mean ± SD and analyzed with the Statistical Package for the Social Sciences (SPSS Release 18.0, SPSS Inc., Chicago, IL, USA). Differences were evaluated using a two-way analysis of variance (ANOVA), followed by Bonferonni posttest. A P-value less than 0.05 was considered as statistically, significantly different.
Results
Fe3O4-MNP enhanced the effect of GA on SMMC-7721 cells
We noted that Fe3O4-MNP at less than 20 μg/mL concentration had no obviously effect on SMMC-7721 proliferation (P>0.05) (Figure 3). GA enhanced the inhibition of proliferation on SMMC-7721 cells in a dose manner. The IC50 value after incubation with GA for 48 hours was 2.40±0.11 μmol/L (P<0.05). Then we investigated the combination of 20 μg/mL Fe3O4-MNP with different concentrations of GA to find the optimal combination to affect SMMC-7721 cells. It was observed that the addition of Fe3O4-MNP could indeed increase the inhibition of SMMC-7721 cells with GA, and 20 μg/mL Fe3O4-MNP could decrease the IC50 value of GA to 1.29±0.07 μmol/L for 48 hours (P<0.05) (Figure 4).
Figure 3.
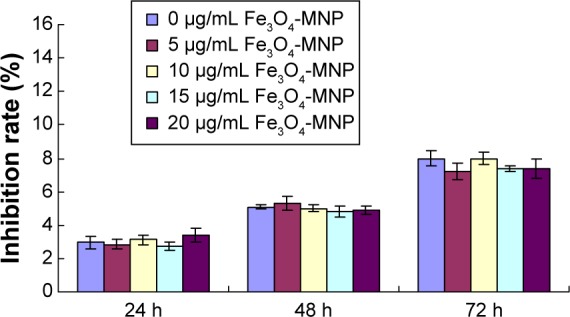
Inhibitory effects of the different concentrations of Fe3O4-MNP on growth of SMMC-7721 cells by MTT assay.
Abbreviations: Fe3O4-MNP, magnetic nanoparticles of Fe3O4; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; h, hours.
Figure 4.
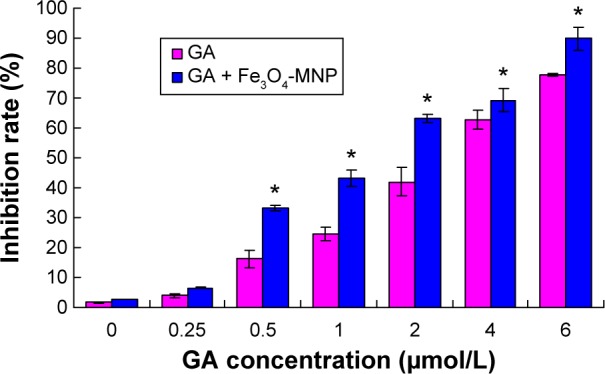
Inhibitory effects of the combination of 20 μg/mL Fe3O4-MNP with different concentrations of GA on growth of SMMC-7721 cells by MTT assay for 48 h.
Note: *P<0.05.
Abbreviations: Fe3O4-MNP, magnetic nanoparticles of Fe3O4; GA, gambogic acid; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; h, hours.
Enhancement of GA-induced apoptosis in SMMC-7721 cells
SMMC-7721 cells treated with 20 μg/mL Fe3O4-MNP (6.25%±0.58%) had no significant changes compared with the control group (6.57%±0.91%) (P>0.05). The apoptotic percentage of SMMC-7721 cells induced by 0.5 μmol/L GA for 48 hours was 20.05%±0.87% (P<0.05), while the combination of GA and Fe3O4-MNP (20 μg/mL) could obviously increase the percentage to 39.62%±2.10% (P<0.05) (Figures 5 and 6).
Figure 5.
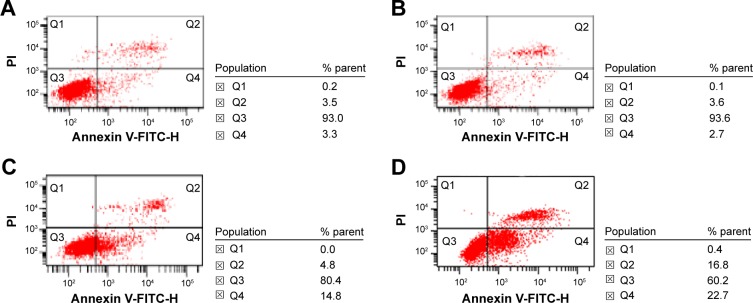
Effect of Fe3O4-MNP on GA-induced apoptosis in SMMC-7721 cells for 48 h.
Notes: (A) Control, (B) 20 μg/mL Fe3O4-MNP, (C) 0.5 μmol/L GA, and (D) 20 μg/mL Fe3O4-MNP + 0.5 μmol/L GA. Q1, mechanical injury cells; Q2, late apoptotic cells; Q3, live cells; Q4, early apoptotic cells.
Abbreviations: Fe3O4-MNP, magnetic nanoparticles of Fe3O4; GA, gambogic acid; h, hours; PI, propidium iodide; FITC, fluorescein isothiocyanate.
Figure 6.
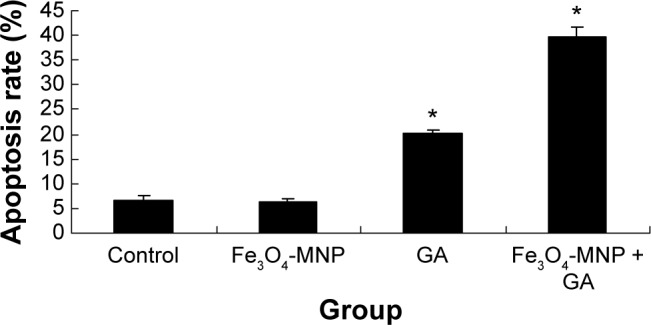
Bar chart of the apoptosis rate in different groups.
Notes: *P<0.05. Apoptosis rate contains early apoptosis (Q4) and late apoptosis (Q2).
Abbreviations: Fe3O4-MNP, magnetic nanoparticles of Fe3O4; GA, gambogic acid.
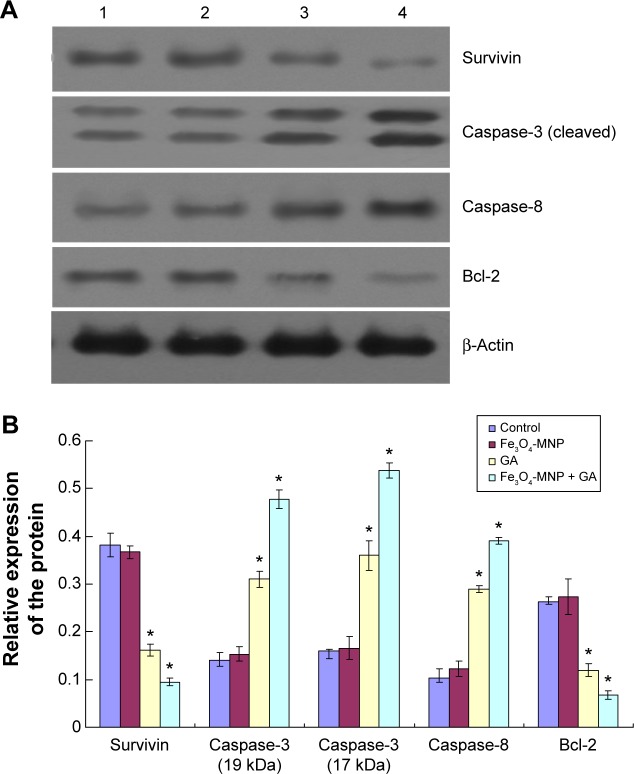
Effects of GA and GA + Fe3O4-MNP on the proteins of survivin, caspase-3, caspase-8, and Bcl-2
The protein level of caspase-3 and caspase-8 in cells treated with 0.5 μmol/L GA (lane C) was obviously elevated, compared with the control group (lane A) (P<0.05). Furthermore, it being upregulated by GA (0.5 μmol/L) with Fe3O4-MNP (20 μg/mL) in SMMC-7721 cells (lane D) was more than that exposed to GA alone (lane C) (P<0.05). Reversely, compared with the control group (lane A), the protein expression of survivin and Bcl-2 was inhibited significantly in SMMC-7721 cells treated with 0.5 μmol/L GA (lane C) (P<0.05). Moreover, the expression level of survivin and Bcl-2 in cells after co-treatment for 48 hours (lane D) was lower than that treated with GA alone (lane C) (P<0.05). There was no statistical difference between the Fe3O4-MNP (20 μg/mL) group and the control group (Figure 7).
Figure 7.
(A) Effect of GA and Fe3O4-MNP on the protein expression level of survivin, caspase-3, caspase-8 and Bcl-2 in SMMC-7721 cells for 48 h. (1) Control, (2) 20 μg/mL Fe3O4-MNP, (3) 0.5 μmol/L GA, and (4) 20 μg/mL Fe3O4-MNP + 0.5 μmol/L GA. (B) Bar graph showing Western blot analysis of survivin, caspase-3, caspase-8, and Bcl-2 in SMMC-7721 cells for 48 h.
Notes: Images were analyzed using Image J. β-Actin was used as an internal standard. The different levels of significance was indicated as *P<0.05.
Abbreviations: GA, gambogic acid; Fe3O4-MNP, magnetic nanoparticles of Fe3O4; h, hours.
Discussion
Primary HCC is one of the most common malignant tumors, especially prevalent in Saharan Africa and Southeast Asia. Effective chemotherapy with standard agents in the treatment of HCC is often accompanied with serious adverse effects causing patients hard to be tolerated; this is one of the most important reasons leading to the failure of chemotherapy. Seeking HCC therapeutic agents with high effect and low toxicity has become one of the main challenges for most pharmaceutical researchers to find or synthesize novel antitumor agents. GA, an apoptotic inducer, can selectively induce tumor cell death without toxicity on normal tissue, which offers a unique prospect for the development of new antitumor medicine.8,9
Many polymer nanoparticles have been introduced as drug delivery systems.10,11 At present, Fe3O4 is the most common material of nanoparticles with low toxicity, magnetic properties, and metabolizable body.12–14 The present study is aimed to demonstrate the potential synergistic effects of Fe3O4-MNP and GA on apoptosis induction in SMMC-7721 cells. The data of MTT assay showed that Fe3O4-MNP at less than 20 μg/mL concentration had no obvious influence on the multiplication of SMMC-7721 cells but decreased the IC50 value of GA. These results confirmed a good biocompatibility of Fe3O4-MNP and also demonstrated that the combination of GA with Fe3O4-MNP exerted a potent cytotoxic effect on SMMC-7721 cells. Our observations indicated that Fe3O4-MNP could promote GA-induced apoptosis in SMMC-7721 cells.
Anti-apoptosis could avoid cancer cell being killed by chemotherapy drugs. The Bcl-2 protein family plays a key role in the anti-apoptosis process.15 Caspase-3 and caspase-8 are the ultimate factors in the apoptotic process.16 The caspase-3 precursor could not be activated if the Bcl-2 is overexpressed. The caspase-3 expression decreases, and apoptosis pathway is blocked.17 GA combined with Fe3O4-MNP dramatically upregulated caspase-3 but downregulated the bcl-2 expression of SMMC-7721 cells, which supports the promotion of GA-induced apoptosis by Fe3O4-MNP with the increased expression level of proteins.
Acknowledgments
The research was supported by Key Department of Jiangsu Medicine, grant number: 2012-12, National Natural Science Foundation of China, grant numbers: 81170492, 81370673, and Key Medical Project of Jiangsu Province, grant number: BL2014078.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhao L, Guo QL, You QD, Wu ZQ, Gu HY. Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 Cells. Biol Pharm Bull. 2004;27(7):998–1003. doi: 10.1248/bpb.27.998. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Lei Y, Yuan P, et al. ROS-mediated autophagy induced by dysregulation of lipid metabolism plays a protective role in colorectal cancer cells treated with gambogic acid. PLoS One. 2014;9(5):e96418. doi: 10.1371/journal.pone.0096418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu JY, Zhou M, Yang JO, et al. Gambogic acid induces mitochondria-dependent apoptosis by modulation of Bcl-2 and Bax in mantle cell lymphoma JeKo-1 cells. Chin J Cancer Res. 2013;25(2):183–191. doi: 10.3978/j.issn.1000-9604.2013.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo GX, Cai J, Lin JZ, et al. Autophagy inhibition promotes gambogic acid-induced suppression of growth and apoptosis in glioblastoma cells. Asian Pac J Cancer Prev. 2012;13(12):6211–6216. doi: 10.7314/apjcp.2012.13.12.6211. [DOI] [PubMed] [Google Scholar]
- 5.Duan D, Zhang B, Yao J, et al. Gambogic acid induces apoptosis in hepatocellular carcinoma SMMC-7721 cells by targeting cytosolicthioredoxin reductase. Free Radic Biol Med. 2014;69:15–25. doi: 10.1016/j.freeradbiomed.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Lin BL, Shen XD, Cui S. Application of nanosized Fe3O4 in anticancer drug carriers with target-orientation and sustained-release properties. Biomed Mater. 2007;2(2):132–134. doi: 10.1088/1748-6041/2/2/011. [DOI] [PubMed] [Google Scholar]
- 7.Li K, Chen B, Xu L, et al. Reversal of multidrug resistance by cisplatin-loaded magnetic Fe3O4 nanoparticles in A549/DDP lung cancer cells in vitro and in vivo. Int J Nanomedicine. 2013;8:1867–1877. doi: 10.2147/IJN.S43752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Yang L, You QD, et al. Differential apoptotic induction of gambogic acid, a novel anticancer natural product, on hepatoma cells and normal hepatocytes. Cancer Lett. 2007;256(2):259–266. doi: 10.1016/j.canlet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Guo QL, Zhao L, You QD, Wu ZQ, Gu HY. Gambogic acid inducing apoptosis in human gastric adenocarcinom SGC-7901 cells. Chin J Nat Med. 2004;2(2):106–110. [Google Scholar]
- 10.Wen J, Jiang S, Chen Z, et al. Apoptosis selectively induced in BEL-7402 cells by folic acid-modified magnetic nanoparticles combined with 100 Hz magnetic field. Int J Nanomedicine. 2014;23(9):2043–2050. doi: 10.2147/IJN.S60457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammadi-Samani S, Miri R, Salmanpour M, Sotoudeh S, Erfani N. Preparation and assessment of chitosan-coated super paramagnetic Fe3O4 nanoparticles for controlled delivery of methotrexate. Res Pharm Sci. 2013;8(1):25–33. [PMC free article] [PubMed] [Google Scholar]
- 12.Sato A, Itcho N, Ishiguro H, et al. Magnetic nanoparticles of Fe3O4 enhance docetaxel-induced prostate cancer cell death. Int J Nanomedicine. 2013;8:3151–3160. doi: 10.2147/IJN.S40766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6(8):865–878. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- 14.Pridgen EM, Langer R, Farokhzad OC. Biodegradable polymeric nanoparticle delivery systems for cancer therapy. Nanomedicine. 2007;2(5):669–680. doi: 10.2217/17435889.2.5.669. [DOI] [PubMed] [Google Scholar]
- 15.Sasson R, Amsterdam A. Stimulation of apoptosis in human granulose cells from in vitro fertilization patients and its prevention by dexamethasone: involvement of cell contact and bcl-2 expression. Clin Endocrinol Metab. 2002;87(7):3441–3451. doi: 10.1210/jcem.87.7.8676. [DOI] [PubMed] [Google Scholar]
- 16.Brauchi S, Cea C, Farias JG, Baciqalupo J, Rayes JG. Apoptosis induced by prolonged exposure to odorants in cultured cells from rat olfactory epithelium. Brain Res. 2006;1103(1):114–122. doi: 10.1016/j.brainres.2006.05.072. [DOI] [PubMed] [Google Scholar]
- 17.Belaud-Rotureau MA, Leducq N, Macouillard Poulletier de Gannes F, et al. Early transitory rise in intraellular PH leads to Bax conformation change during ceramide-induced apoptosis. Apoptosis. 2005;5(6):551–560. doi: 10.1023/a:1009693630664. [DOI] [PubMed] [Google Scholar]