Summary
With the explosion of genome-wide studies of regulated transcription, it has become clear that traditional definitions of enhancers and promoters need to be revisited. These control elements can now be characterized in terms of their local and regional architecture, their regulatory components including histone modifications and associated binding factors and their functional contribution to transcription. This review discusses unifying themes between promoters and enhancers in transcriptional regulatory mechanisms.
Recent genome-wide studies have significantly advanced our understanding of the genomic architecture that underlies gene expression in higher eukaryotes. Integrative analyses of the transcriptome, transcription factor (TF) binding profiles, and epigenomes reveal complex organization of individual transcription units scattered throughout the genome and the causal relationship among the regulatory DNA sequences, chromatin state, and transcriptional activity. In particular, a considerable amount of data have established that enhancers are not merely a collection of TF binding sites, but also have the capacity to drive transcription independent of their target promoters. This feature of enhancers suggests that they serve more regulatory functions than previously appreciated.
Regulatory DNA elements in eukaryotic gene expression
Transcription of a gene in eukaryotes is a highly complex process that requires precise coordination in the assembly of trans-acting factors through recognition of various types of regulatory DNA sequences. The promoter and the enhancer represent DNA regulatory regions responsible for ensuring proper spatiotemporal expression patterns of eukaryotic genes. The promoter generally refers to a DNA region that allows accurate transcription initiation of a gene (Smale and Kadonaga, 2003). The core promoter is a minimal stretch of DNA sequences, (e.g., the TATA box, initiator, and downstream core promoter element) surrounding the transcription start site that directly interact with the components of basal transcription machinery including RNA polymerase II (RNAPII). Although the DNA sequences or motifs comprising the core promoter region for individual genes can be structurally and functionally diverse, its universal role is thought to drive accurate transcription initiation (Smale and Kadonaga, 2003). Transcription factors that bind ~100–200 bp upstream of the core promoter can increase the rate of transcription by facilitating the recruitment or assembly of the basal transcription machinery onto the core promoter, or by mediating the recruitment of specific distal regulatory DNA sequences to the core promoter (Akbari et al., 2008).
These distal sequences, known as enhancers, activate or increase the rate of transcription from the target gene promoter independent of their position and orientation with respect to target genes (Maniatis et al., 1987). In multicellular organisms, enhancers are primarily responsible for the precise control of spatiotemporal patterns of gene expression. Enhancer elements were initially discovered in the early 1980s in the studies characterizing eukaryotic gene promoters. Functional tests of sea urchin histone gene expression in the Xenopus oocyte identified DNA sequences located upstream of the TATA box motif that positively influence H2A gene transcription, originally termed transcriptional “modulators” (Grosschedl and Birnstiel, 1980). Deletion of the modulator resulted in 15 – 20 fold decrease in H2A gene expression. Interestingly the modulator activity was retained even when its DNA sequence was inverted. Similarly, the tandem 72 bp DNA repeats located upstream of viral SV40 early gene was found to be indispensible for SV40 early gene expression (Benoist and Chambon, 1981). Shortly after those initial observations, a series of studies on the SV40 enhancer established the conceptual framework for defining enhancers as follows (Atchison, 1988; Banerji et al., 1981; Fromm and Berg, 1982, 1983; Khoury and Gruss, 1983; Moreau et al., 1981): i) Enhancers increase transcription of a linked gene from its correct initiation site specified by the core promoter, ii) Enhancer activity is independent of orientation relative to its target gene, iii) Enhancers can function over a long distance and independent of their position relative to their target gene, iv) Enhancers can function with a heterologous promoter, v) Enhancers exhibit DNase I hypersensitivity (HS), which reflects a less compacted chromatin state as a result of the binding of various transcription factors. Although these properties were defined more than three decades ago, they are still widely used to classify enhancers.
Subsequent studies identified the first mammalian cellular enhancer, which is required for efficient expression of the immunoglobulin (Ig) heavy-chain gene (Banerji et al., 1983; Gillies et al., 1983; Neuberger, 1983). Importantly, the Ig enhancer studies provided the first evidence demonstrating that enhancer activity can be tissue- or cell type-specific. When various cell lines were tested, Ig enhancer activity was observed only in lymphocyte-derived cell lines (Banerji et al., 1983; Gillies et al., 1983). Since then, a variety of cell type- or developmental stage-specific enhancers have been determined to regulate the expression of genes in higher organisms (Muller et al., 1988). Transcriptional activation of yeast genes was also shown to be mediated by enhancer-like sequences, known as upstream activation sequences (UASs) although their distances from the core promoters are much shorter (within a few hundred base pairs) than the typical distances between enhancers and promoters in mammals (Guarente, 1988). These results led to the realization that enhancer activity is the primary mechanism for determining the spatiotemporal gene expression pattern in eukaryotes.
RNAPII association at enhancers and locus control regions
The ability to recruit RNAPII and initiate transcription has generally been considered the most unique property of promoters. However, even before the genomics era, several studies found that RNAPII can be directly recruited to enhancers upon transcriptional induction, potentially serving as a regulatory checkpoint for RNAPII delivery to the target promoter. Interestingly, an early study of the SV40 enhancer found that in the absence of any known promoter sequence the 72 bp DNA repeats can also “promote” gene expression, although this was deemed to be inefficient, (Benoist and Chambon, 1981; Moreau et al., 1981). This finding suggested the possibility that the 72 bp sequence might serve as a general entry site for a component of the transcription machinery such as RNAPII that could then track along the DNA to a transcription initiation site (Moreau et al., 1981). Another proposed mechanism that may not be mutually exclusive with the RNAPII tracking model is the chromatin remodeling effect. As various chromatin modifying enzymes such as histone acetyltransferases and methyltransferases can be part of the RNAPII transcription complex (Cho et al., 1998; Gerber and Shilatifard, 2003), transcription initiated from the enhancer proceeding across the intervening regions between the enhancer and the target promoter might be responsible for establishment and/or maintenance of an active chromatin conformation required for efficient gene transcription.
Initial studies of enhancer identification and characterization were carried out by transient transfection experiments, which means that enhancer activity may be subject to position-effect variegation depending on the chromatin configuration at the genomic site of integration. However, a study of a transgene containing the human β-globin locus discovered that five DNase-I hypersensitive sites scattered in a ~70 kb region surrounding the β-globin gene was sufficient to overcome the positional effect (Grosveld et al., 1987). These cis-regulatory regions (e.g., enhancers) conferring activation of a linked gene in a tissue-specific, copy-number-dependent manner, independent of its position of integration was collectively termed a locus control region (LCR) (Orkin, 1990). Notably, transcription activity was detected at enhancers located within the β-globin LCR region and through the intervening DNA into the globin genes (Ashe et al., 1997; Routledge and Proudfoot, 2002; Tuan et al., 1992). These LCR-driven intergenic transcripts are relatively short (< 3 kb), remain in discrete foci in the nucleus, and do not encode proteins (Ling et al., 2004). Transcription predominantly occurs toward the downstream globin genes, but was independent of the orientation, position, and distance of the enhancers with respect to the gene (Kong et al., 1997; Routledge and Proudfoot, 2002). RNAPII recruitment and transcription activity have also been observed in other LCRs, including those that control expression of major histocompatibility complex (MHC) class II in antigen-presenting immune cells and pituitary-specific expression of the human growth hormone (hGH) gene (Ho et al., 2006; Masternak et al., 2003). Interestingly, insertion of an exogenous RNAPII termination sequence within the hGH-LCR blocked hGH regulation, suggesting that transcription through the LCR domain is a functionally important event.
In both the human and murine β-globin gene loci, RNAPII interacts with the LCR, but not directly with the β-globin gene prior to erythroid differentiation; whereas it is associated with both in differentiated erythroid cells (Levings et al., 2006; Vieira et al., 2004). In an in vitro assay using nuclear extracts from MEL cells, RNAPII and other basal transcription factors associated with immobilized LCR templates could be transferred to a β-globin gene template, which was facilitated by an erythroid transcription factor NF-E2 (Vieira et al., 2004). Although performed in vitro, these results suggest a model where the β-globin LCR functions to assemble and hold the RNAPII transcription complex for timely delivery to the β-globin gene to ensure the developmentally stage-specific expression. Furthermore, blocking transcription elongation between the LCR and the promoter by the insertion of a transcription terminator sequence significantly decreased the β-globin mRNA level, suggesting that the β-globin LCR facilitates a tracking and transcription mechanism (Ling et al., 2004). A similar mechanism has been proposed for other LCRs and enhancers (Ho et al., 2006; Wang et al., 2005). In a contrasting model, transfer of the RNAPII machinery from the α-globin LCR to the promoter appears to be mediated by formation of a DNA loop between the LCR and the promoter as no RNAPII signal is detected in the intervening DNA between the LCR and the promoter (Vernimmen et al., 2007).
Genome-wide architecture of enhancers
These initial insights into the complex roles for enhancers and LCRs set the stage for thinking about regulatory elements in a more global manner. Early genome-wide studies identified RNAPII binding at intergenic loci, which suggested the existence of enhancer-like sequences across the genome; however, there were questions regarding the functional relevance of such RNAPII occupancy (Barrera et al., 2008; Brodsky et al., 2005; Carroll et al., 2006; Heintzman et al., 2007). Moreover, it was difficult to classify RNAPII binding sites as possible enhancer or un-annotated promoters of a protein-coding gene by the virtue of RNAPII association alone.
It became clear that additional criteria would be needed to identify enhancers. Given their association with transcription factors, computational analysis of TF binding motifs combined with the assessment of evolutionary conservation within the DNA was used as a popular approach in identifying enhancers [reviewed in (Aerts, 2012)]. More recently, chromatin-immunoprecipitation-based analysis of TF binding in vivo (e.g., ChIP-chip and ChIP-seq) has been widely used to determine experimentally actual TF binding sites in vivo. This approach revealed that only a small fraction of TF binding motifs are actually bound by TFs in vivo in a given tissue and/or stage (Consortium et al., 2007). TF binding per se does not signal a functional outcome. Functional activation requires recruitment of additional cofactors or mechanisms involving a combinatorial coordination of multiple TFs. Therefore, analysis of evolutionarily conserved TF motifs or TF binding alone has a limited power for identification and prediction of functional enhancers [see also (Kellis et al., 2014) for review].
Transcriptional coactivators p300 and CBP interact with a large number of transcriptional activators and the general transcription machinery including RNAPII. Moreover, both p300 and CBP display acetyltransferase activity toward the tails of histones localized near cis-regulatory regions, which is thought to create a transcriptionally permissive chromatin structure. Therefore, although not perfect, genome-wide analysis of p300/CBP binding sites has been commonly used as a method for identifying enhancer elements in vivo without having to investigate individual TFs (May et al., 2012; Visel et al., 2009)
A complementary approach in identifying enhancers takes advantage of their chromatin accessibility. The assembly of various TF complexes at cis-regulatory regions is considered to compete with stable association of nucleosomes. As a result, active enhancers and promoters have reduced nucleosome density and display hypersensitivity to DNase I digestion. This feature of chromatin accessibility has been utilized in next generation sequencing-based techniques such DNase-seq, FAIRE-seq, and ATAC-seq (Boyle et al., 2008; Buenrostro et al., 2013; Giresi et al., 2007) to identify enhancers without any prior knowledge of TF binding motifs or factor binding. Although not sufficient to pinpoint cell type-specific enhancers due to its indiscriminate nature, this method can be very useful for enhancer characterization when combined with other mapping techniques.
An increasing number of epigenomic studies have illustrated that the chromatin of metazoan genomes is organized into modular domains that represent unique chromatin states formed by a combination of multiple post-translational modifications on histones within the nucleosomes (Consortium et al., 2012; Ernst et al., 2011). For example, nucleosomes within enhancer regions contain histone variants H3.3 and H2A.Z (Goldberg et al., 2010; Henikoff et al., 2009; Jin et al., 2009). These nucleosome variants are deposited into enhancer regions in a replication-independent manner and are more sensitive to high salt than canonical nucleosomes. In contrast, nucleosomes flanking TF-bound sites are stable and undergo various histone modifications that are distinctive to each functional domain and across cell types, and correlate with transcriptional outputs (Consortium et al., 2012; Heintzman et al., 2009; Heintzman et al., 2007; Hon et al., 2009; Visel et al., 2009). Importantly, such chromatin modifications combined with other measures (chromatin accessibility and TF binding) have proven a useful barometer for active enhancers. Enhancers of active genes generally display a high level of mono- or di-methylation on H3 lysine 4 (H3K4me1/2), but are low or devoid of H3K4me3 whereas promoter sequences show the opposite pattern. In addition to H3K4me1/2, mutually exclusive modifications on H3K27 residues co-segregate with active or inactive/poised enhancers (Creyghton et al., 2010; Rada-Iglesias et al., 2011). Active enhancers are enriched in the H3K27ac mark, a major substrate for the histone acetyltransferase p300/CBP (Jin et al., 2011; Tie et al., 2009), while poised enhancers are associated with H3K27me3, a mark enriched in Polycomb (PcG)-associated and transcriptionally repressed regions (Rada-Iglesias et al., 2011). Additionally, H3K27me3 also co-exists with the active promoter mark H3K4me3 in the promoters of developmentally silenced genes in ES cells, known as poised/bivalent promoters (Bernstein et al., 2006).
Although enhancers share common structural and functional features as described above, individual enhancers widely differ in the enrichment levels of TF and enhancer-specific histone modifications. A set of recent studies inspected enhancers based on the quantitative difference in the level of Mediator complex binding or H3K27ac marks, and found that enhancers are often clustered in large domains, termed super-enhancers. Typically a few hundred super-enhancers are present in a given cell type, and are often located near cell-type specific genes or the genes that control the biological processes that define the identities of the cell types (Hnisz et al., 2013; Loven et al., 2013; Whyte et al., 2013). Consistently, a strong enrichment of disease-associated non-coding variants has been observed within super-enhancers (Hnisz et al., 2013). Each super-enhancer represents a functional cluster of multiple enhancer units that communicate with each other physically and functionally, and provide a platform where various signaling pathways converge to robustly regulate genes that control cell identity during development and tumorigenesis (Hnisz et al., 2015). With that operational definition, super-enhancers appear to be highly analogous to the “regulatory archipelago” described at the HoxD locus (Montavon et al., 2011). Although more analysis will be required to establish whether or not super-enhancers reflect a novel paradigm in gene regulation, their identification in each cell type would, at least, be very useful for the characterization of the cell-type-specific regulatory network.
From enhancer sequences to enhancer RNAs
In 2010, two independent studies reported that direct RNAPII recruitment and transcription are genome-wide features of functionally active enhancers. In neurons, a combination of enhancer markers (high levels of H3K4me1 overlapped with CBP binding, but with no or low H3K4me3) was used to identify ~12,000 neuronal enhancers that mediate transcription induction upon neuronal activation by membrane depolarization (Kim et al., 2010). Interestingly ~ 25% of the neuronal enhancers also exhibited a significant level of RNAPII binding and produced RNA transcripts. These enhancers RNAs (eRNAs) are dynamically regulated by neural activity with their levels positively correlating with mRNA levels of nearby protein-coding genes. The majority of eRNAs characterized in neurons are short (< 2 kb), lack polyadenylated tails and do not appear to be spliced. Notably, global profiling showed that eRNAs are transcribed bi-directionally from the center of enhancers where CBP and RNAPII are bound. Another study discovered eRNAs (originally referred in the study as inducible upstream extragenic transcripts) in endotoxin-stimulated primary macrophages (De Santa et al., 2010). RNAPII ChIP-seq analysis identified 4855 extragenic RNAPII binding sites and ~70% of them showed an enhancer-like chromatin signature (high levels of H3K4me1 with low or no H3K4me3). Many of these extragenic enhancers produce eRNAs upon LPS stimulation. Unlike neuronal eRNAs, several macrophage eRNAs were shown to be produced from uni-directional transcription and to be polyadenylated without being spliced.
Since these initial discoveries, eRNAs have been found in many mammalian cell types including embryonic stem cells, suggesting that eRNA synthesis is a universal cellular mechanism [reviewed in (Lam et al., 2014)]. Super-enhancers exhibit a much higher level of RNAPII binding and eRNA transcription than typical enhancers (Hah et al., 2015; Hnisz et al., 2013). Multiple eRNAs are generated within super-enhancers with a striking correlation in their expression patterns, which could imply that each super-enhancer might form a single regulatory module (Hah et al., 2015). Importantly, a recent study has identified the RNAPII-associated complex, Integrator, as the molecular machine involved in the 3′-end processing of eRNAs at enhancers and super-enhancers (Lai et al., in press).
As greater numbers of eRNAs have been identified, we’ve gained more detailed insights into their properties and regulation. The majority, although not all, of eRNAs in the nucleus lack polyadenylated tails (Consortium et al., 2012; Derrien et al., 2012; Djebali et al., 2013; Harrow et al., 2012). A genome-wide study in murine CD4+ CD8+ thymocytes correlated non-polyadenylated and polyadenylated eRNAs with bi-directionally and uni-directional transcription, respectively, although the functional implication of this dichotomy is not known (Koch et al., 2011; Natoli and Andrau, 2011). Moreover, eRNA-producing enhancers are cell type-specific and associated with a chromatin signature unique to functionally active enhancers, including H3K4me1, H3K27 acetylation and H3K79 dimethylation along with RNAPII binding (Djebali et al., 2013). A genome-wide chromosomal interaction study in several human cell lines further demonstrated that eRNA-producing enhancers are preferentially engaged in an interaction with the proximal promoters (Sanyal et al., 2013). Another notable feature of eRNAs is the timing of their expression relative to mRNA upon stimulus-dependent induction. In many different cell types, eRNA transcription marks the earliest response in the wave of transcriptional change when cells undergo a state change in response to environmental or developmental cues (Arner et al., 2015; De Santa et al., 2010; Hah et al., 2013; Hsieh et al., 2014; Schaukowitch et al., 2014).
Promoter vs. Enhancer – a new comparison in the genomic era
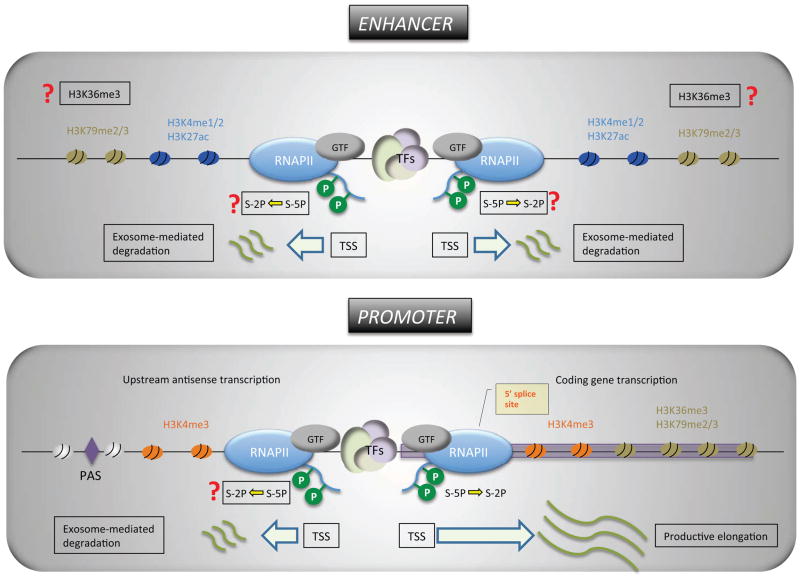
Recent genome-wide evidence of transcribing enhancers observed in a wide range of cell types argues that the conventional definitions of the promoter and the enhancer must be revised. The roles of promoters and enhancers in transcription have been thought to be distinct; however, these two regulatory elements are highly interrelated and show noticeable similarities in structure and function. As summarized below, both the promoter and the enhancer exhibit common structural and functional features that have not been previously appreciated (Figure 1).
Figure 1. A contemporary view on promoters and enhancers.
Features of promoters include: Transcription initiation in the sense and anti-sense direction is mediated by the transcription machinery assembled independently onto its own core promoter. Although not shown here, convergent transcription has been observed at the promoters of weakly expressed genes. H3K4me3 is highly enriched at the promoter regions. Enhancer-like chromatin signatures (H3K4me1 and H3K27ac) and the Tyr-1P form of the RNAPII have also been observed near the upstream anti-sense TSSs. Polyadenylation sites are enriched near the 3′ end of the upstream anti-sense RNAs and mediate the exosome-dependent degradation of the antisense RNAs. 5′ splice sites are only present in the coding gene, and might contribute to the productive elongation of sense mRNA transcripts through the binding of the U1 splicing complex, which blocks PAS-mediated early termination. The Ser-5P form of RNAPII is engaged in upstream anti-sense transcription, but it is not known if Ser-2P of RNAPII occurs during the elongation of anti-sense RNA.
Features of enhancers include: Like the promoter, the enhancer recruits the general transcription factors (GTF) including RNAPII and initiates transcription at defined sites. Enhancer-driven transcription typically exhibits more prominent bi-directionality than that stemming from the promoter. H3K4me1/2 is commonly enriched at enhancers. Functionally active enhancers also exhibit a high level of H3K27 acetylation whereas poised or inactive enhancers are marked by H3K27me3. Ser-5P and Tyr-1P forms of the RNAPII have been observed. It is not clear whether or not Ser-2P RNAPII and H3K36me3 marks are present at active enhancers. 5′ splice site sequences are not enriched near the regions surrounding enhancers. Both strands of enhancer RNAs appear to be degraded by the exosome, although it is not known whether it is mediated by the PAS-dependent mechanism.
Shared local structure
Both promoters and enhancers display DNase I hypersensitivity, which results from depletion of nucleosomes. This local structure arises because both regulatory regions are composed of binding sites for TFs, which exclude nucleosomes. However, whether or not there is any distinguishable difference in TF binding site composition between the regions is somewhat debatable. Initial genome-wide studies suggested that promoters and enhancers differ in the composition of binding sites (Rada-Iglesias et al., 2011; Shen et al., 2012; Thurman et al., 2012). However, recent FANTOM5 cap analysis gene expression (CAGE) studies argue that the difference in binding site composition might simply result from the fact that enhancers are largely devoid of CpG islands (CGI) and repeats resembling non-CGI promoters (Andersson et al., 2014). Consistently some older studies showed that interacting promoter-enhancer pairs often harbor common TF binding sites (Bienz and Pelham, 1986; Bohmann et al., 1987; Parslow et al., 1987).
Consistent histone modification patterns
Although the local ratio of H3K4me3/me1 has been widely used as means to distinguish enhancers and promoters, recent studies argue that the three H3K4 methylation states (H3K4me1/2/3) simply reflect dynamic changes in transcription activities of both the promoters and enhancers rather than representing static and intrinsic features of individual regulatory elements. The majority of enhancers simply show a low H3K4me3/me1 ratio as their transcription level is generally lower than promoter-driven transcription. However, H3K4me3 is often observed at functionally active enhancers and the H3K4me3/me1 ratio positively correlates with transcription level, independent of transcript stability (Core et al., 2014; Koch et al., 2011; Pekowska et al., 2011).
Functional interchangeability
The notion that promoters and enhancers functionally overlap was initially supported by the finding that the proximal promoter region of mouse metallothionein-I (mMT-I) gene, when inserted downstream of a rabbit β-globin test gene, could enhance β-globin transcription upon metal ion stimulation, thus acting as an inducible enhancer (Serfling et al., 1985). Moreover, a chromosomal interaction study found that promoters frequently associate with other promoters through space analogous to well-characterized promoter-enhancer interactions, which could imply an enhancer-like function of the promoter in transcription (Li et al., 2012). Recently, intragenic enhancers were shown to frequently function as alternative tissue-specific promoters producing a class of abundant, spliced, multi-exonic poly(A)+ RNAs (meRNAs) reflecting the host gene’s structure (Kowalczyk et al., 2012). These examples collectively support the notion that the enhancers and promoters not only share many of the similar architectural features (nucleosome hypersensitivity and chromatin marks) but also may be functionally interchangeable.
Common mechanisms to control RNA synthesis
Similar to promoters, RNAPII and general transcription factors (GTFs) are assembled on enhancers and initiate transcription (Koch et al., 2011; Natoli and Andrau, 2011). The C-terminal domain (CTD) of RNAPII is composed of multiple heptapeptide repeats (YSPTSPS) and undergoes differential phosphorylation as the transcription cycle progresses. While, unphosphorylated RNAPII enters the pre-initiation complex, escape from the promoter, is highlighted by phosphorylation of the Ser-5 residues of the CTD, and entry of the RNAPII into productive elongation is coordinated by a wave of Ser-2 phosphorylation. Both un-phosphorylated and Ser-5 phosphorylated forms of RNAPII are also observed at enhancers. Moreover, tyrosine 1 phosphorylation of the RNAPII CTD has been observed with antisense promoter transcription and active enhancers in mammalian cells (Descostes et al., 2014). However, the elongation-specific form of RNAPII (Ser-2 phosphorylated) as well as the H3K36me3 mark, both of which are normally seen across the coding regions of actively transcribing genes, have not been readily detected in the eRNA transcribing areas (Kaikkonen et al., 2013; Koch et al., 2011; Natoli and Andrau, 2011). On the other hand, several studies observed H3K79me2/3 marks over transcribed enhancer regions, an additional coding region-specific modification whose levels are highly correlated with transcription activity (Bonn et al., 2012; Djebali et al., 2013).
At this point, it is not clear whether the lack of elongation-specific marks (H3K36me3 and Ser-2 phosphorylation of RNAPII) at enhancers reflect a fundamentally different transcription mechanism between enhancers and promoters or if the eRNA regions being transcribed are simply not long enough to sufficiently accumulate those marks, which are known to be enriched near the 3′ end of genes. Alternatively, the levels of eRNA transcription might not be high enough to observe the enrichment of these elongation-specific features as their levels are generally correlated with transcription output.
Bi-directional transcription
Bi-directionality is a striking feature of eRNA transcription that has been documented at many enhancers. However the majority of mammalian promoters also drive divergent transcription, resulting in the production of short antisense ncRNAs (known as uaRNAs, PROMPTs, or promoter upstream transcripts) from upstream promoter regions in addition to sense mRNAs (Core et al., 2008; Preker et al., 2008; Seila et al., 2008). Both eRNAs and promoter upstream antisense transcripts are relatively unstable possibly due to exosome-mediated degradation (Andersson et al., 2014; Flynn et al., 2011). Genome-wide analyses integrating nascent transcript mapping, DNase I hypersensitive sites, nucleosome positions, and binding profiles of various TFs and histone modifications have corroborated the shared architecture of transcription initiation between enhancers and promoters. Both enhancers and promoters exhibit similar frequencies of canonical core promoter elements, highly positioned flanking nucleosomes, and tight average spacing (~110bp) between each pair of divergent TSSs. Divergent transcription at promoters and enhancers is mediated by independent RNAPII transcription complexes assembled at each TSSs, which is intrinsically configured by underlying core elements as well as TF binding motifs enriched near both sense and anti-sense TSSs (Core et al., 2014; Duttke et al., 2015; Scruggs et al., 2015). Moreover, elevated levels of TF binding and enhancer-like chromatin signatures (e.g., high levels of H3K4me1 and H3K27ac) were observed near the anti-sense TSSs located upstream of highly transcribed sense TSSs (Scruggs et al., 2015). Intriguingly, a nucleotide-resolution mapping analysis of RNAPII position by native elongating transcript sequencing (NET-seq) has revealed that the promoters of genes expressed at a low level in human HeLa or HEK293T cells drive convergent transcription, in which antisense transcription originates downstream of the sense TSS (Mayer et al., 2015). It is not known whether convergent transcription is also a feature of enhancers.
Regulation of upstream transcription
Computational analysis of promoters showed that the regions where upstream antisense transcription occurs are enriched in polyadenylation sites (PAS), but depleted of potential U1 small nuclear ribonucleoprotein (snRNP) recognition sites, or 5′ splice site-like sequences. This asymmetric feature in functional DNA motifs flanking TSSs was argued to underlie promoter directionality (Almada et al., 2013; Core et al., 2014; Ntini et al., 2013). Transcription of upstream antisense RNAs terminates at the enriched PAS, and the RNAs are then degraded by the exosome whereas the sense transcripts are protected by U1 snRNP, which prevents premature cleavage and polyadenylation (Berg et al., 2012; Kaida et al., 2010). The FANTOM5 CAGE analysis suggested that the eRNAs are also subject to a similar decay mechanism. However, unlike the promoters, the DNA regions flanking enhancers do not show an enrichment of 5′-splice site sequences (Andersson et al., 2014).
The role of enhancer transcription
The defined characteristics of eRNAs – low abundance, low stability, lack of RNA processing such as polyadenylation and splicing, and bi-directionality in transcription – could collectively suggest that eRNAs are the byproduct of enhancer transcription activity with no biological function. This idea of transcriptional noise proposes that excess RNAPII machinery is uniformly associated with physically accessible genomic regions, including enhancer regions, and initiates transcription ‘nonspecifically’ from incorrect sites (Struhl, 2007). In this model, nonspecific transcripts are generally in low abundance as they are rapidly degraded by intrinsic cellular surveillance mechanisms such as nonsense-mediated decay or exosome-mediated degradation (LaCava et al., 2005; Wyers et al., 2005).
However, transcription does not appear to be a random process. For example, there is no transcription activity in poised enhancers, which clearly show chromatin accessibility judged by DNase I hypersensitivity. It was also proposed that enhancers that mediate rapid induction of neural genes in response to membrane depolarization do not transcribe eRNAs unless the enhancer is paired with its target promoter (Kim et al., 2010). However, enhancer transcription initiated from hGH-LCR in the pituitary was independent of the interaction with the target hGH-N promoter (Yoo et al., 2012). Despite this discrepancy in the promoter dependency of eRNA production, it is generally agreed that eRNA transcription occurs only from functionally active enhancers in a regulated manner (Andersson et al., 2014; Core et al., 2014; Creyghton et al., 2010; Hah et al., 2011; Kaikkonen et al., 2013; Kim et al., 2010; Rada-Iglesias et al., 2011). Furthermore, as we have described, both enhancers and promoters share key architectures of transcriptional initiation sites. These features collectively suggest that eRNA synthesis is a regulated process with its transcription initiation fidelity comparable to the promoter, rather than a consequence of random RNAPII transcription initiation from accessible genomic regions; although they do not necessarily prove the functionality of eRNA transcripts (Weingarten-Gabbay and Segal, 2014).
When considering the functional relevance of enhancer transcription, several lines of evidence suggest that the act of eRNA transcription, rather than the eRNA transcript itself, might have a specific biological function. One possibility is that enhancer-promoter pairing or looping is mediated by a tracking mechanism where the enhancer-bound transcription complex is ferried to a specific target promoter via uni-directional RNAPII transcription. Consistently, LCR-driven transcription takes a uni-directional path toward target genes (Ashe et al., 1997; Ho et al., 2006; Ling et al., 2005; Routledge and Proudfoot, 2002), and some eRNAs in T lymphocytes were also shown to be transcribed uni-directionally (Koch et al., 2011; Natoli and Andrau, 2011). However, global profiles of eRNA expression argue that such a simple tracking/scanning mechanism of enhancer-promoter communication might not be general as the majority of enhancer transcription occurs bi-directionally within confined flanking regions not contiguous to the target gene.
Since RNAPII can carry histone-modifying enzymes through interactions with its CTD [see review in (Selth et al., 2010)], RNAPII transcription could be an underlying mechanism for altering the chromatin architecture at enhancers or intervening DNA regions between enhancers and promoters. Indeed, active chromatin modifications such as histone hyperacetylation and DNase I hypersensitivity are often observed near RNAPII transcribed regions (Bulger et al., 2003; Gribnau et al., 2000; Masternak et al., 2003; Travers, 1999). For example, a transcription inhibitor, actinomycin D significantly blocked LPS-induced histone hyperacetylation in the intervening regions between inducible gene promoters and enhancers in macrophages (De Santa et al., 2010). Another study in macrophages showed that TLR4 signaling-induced eRNA transcription precedes a local increase in the level of H3K4me1/2, and the length of eRNAs coincides with the width of the H3K4me1/2-modified region (Kaikkonen et al., 2013). A transcription elongation inhibitor, flavopiridol, but not the eRNA knockdown, significantly reduces the level of H3K4me1/2 at enhancers, suggesting that transcription activity at enhancers, not the eRNA transcript itself, might be important for at least some aspect of enhancer-specific chromatin modification (Kaikkonen et al., 2013). However, flavopiridol treatment in MCF-7 cells did not alter the levels of enhancer-specific histone marks (i.e., H3K4me1 or H3K27ac) (Hah et al., 2013). One potential source for this discrepancy might be due to differences in the stability of the enhancer-specific marks between the two cell types (T cells vs. MCF-7 cells) and/or the mode of stimulus-induced signaling although the aforementioned study in macrophages claimed that the effect of transcription blockers in H3K4me1/2 modifications is also observed in pre-existing enhancers (Kaikkonen et al., 2013). It also needs to be noted that the proposed function of enhancer transcription in the enhancer-specific chromatin landscape does not have to be mutually exclusive with a possibility that the eRNA transcript itself might play a functional role in transcriptional activation.
The role of eRNA transcript
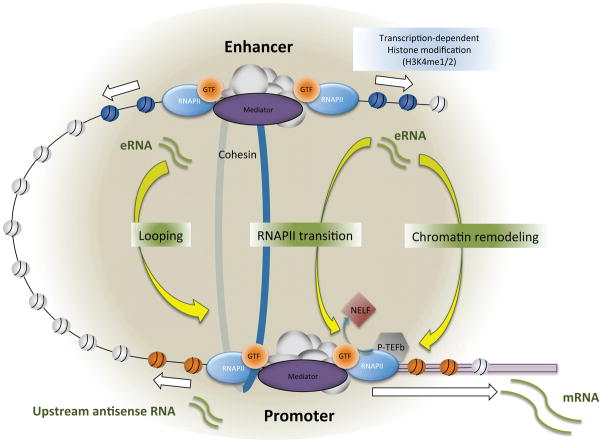
Several recent studies have suggested that the eRNA transcript itself might have an activating role in target gene expression in various cell types (Hsieh et al., 2014; Lam et al., 2013; Li et al., 2013; Melo et al., 2012; Mousavi et al., 2013; NE et al., 2014; Schaukowitch et al., 2014). Knockdown of eRNAs generated from various enhancer regions consistently causes a decrease in the expression of their specific target genes. Multiple mechanisms have been described to underlie the eRNA function. These include the eRNAs regulating enhancer-promoter looping, chromatin remodeling, and early transcription elongation (Figure 2). In human MCF-7 breast cancer cells, several eRNAs expressed from estrogen receptor-α bound enhancers facilitate specific enhancer-promoter interactions in a ligand-dependent manner by recruiting the cohesin complex to the enhancer from which they originated (Li et al., 2013). An eRNA expressed from Kallikrein-related peptidase 3 (KLK3) enhancer, one of the strongest androgen receptor (AR)-bound enhancers in prostate cancer cells, was also shown to facilitate a specific interaction between the KLK3 enhancer and the KLK2 promoter, but in this case by forming a complex with AR and a subunit of the Mediator complex, Med1 (Hsieh et al., 2014). Therefore, chromosomal looping between specific set(s) of enhancers and promoters appears to be a key regulatory step in which both eRNAs and other activating lncRNA can commonly act (Figure 2). By contrast, the eRNA expressed from the MYOD1 core enhancer (CERNA) during the myogenic differentiation of C2C12 skeletal muscle cells had no impact on the enhancer-promoter interaction (Mousavi et al., 2013). Instead, CERNA increased RNAPII occupancy at the promoter region of the MYOD1 gene and subsequent transcription by promoting chromatin accessibility. Although the exact mechanism has not been defined, the chromatin remodeling activity of CERNA is reminiscent of the function of HOTTIP (Wang et al., 2011) (Figure 2).
Figure 2. Mechanisms of enhancer-promoter interactions.
H3K4me1/2 modification at enhancers can be mediated by RNAPII transcription activity. Enhancer RNA is also shown to play a role in various stages of transcription. Looping: The Mediator/Cohesin complex is involved in stable formation of enhancer-promoter looping. Some eRNAs (e.g., ncRNA-a, and eRNAs expressed from oestrogen receptor-α bound enhancers) facilitate the looping through an interaction with the subunit(s) of the Mediator/Cohesin complex. Chromatin remodeling: eRNAs (e.g., CERNA) can also promote transcription by remodeling the chromatin structure such that the accessibility of RNAPII machinery is increased. RNAPII transition: Early RNAPII elongation is another transcription step regulated by eRNAs. eRNAs (e.g., Arc eRNAs) can help RNAPII enter into a productive elongation stage by facilitating transient release of the negative elongation factor, NELF, which causes RNAPII pausing near the TSS.
Early transcription elongation is another step in which eRNAs play a role. RNAPII pausing immediately downstream of the transcription start sites is a widespread regulatory mechanism in higher eukaryotes, which is mediated by negative elongation factor, NELF and DRB sensitivity-inducing factor. By serving as a key rate-limiting step, RNAPII pausing allows the convergence of signaling pathways and is thought to be important for the establishment of permissible chromatin structure as well as rapid and/or synchronous gene expression (Adelman and Lis, 2012). During induction of neuronal immediate early genes, eRNAs contribute to the gene induction in cis by promoting efficient release of NELF from their target gene promoters. eRNAs are rapidly transcribed and destabilize NELF’s association with paused RNAPII by directly binding to the RNA recognition motif present in the NELF-E subunit (Schaukowitch et al., 2014). Knockdown of eRNA blocks transient release of NELF from the promoter during transcription activation and specifically decreases the amount of elongating RNAPII without affecting the RNAPII recruitment step or chromosomal looping between the enhancer and the promoter (Figure 2).
lncRNAs with enhancer-like functions
In parallel with the eRNA studies, an independent study discovered an enhancer-like function for a set of long non-coding RNAs (lncRNAs) in human cell lines, termed ncRNA-activating (ncRNA-a) (Lai et al., 2013; Orom et al., 2010). Knockdown of several lncRNAs in this class invariably reduced expression levels of nearby protein coding genes. A subsequent mechanistic study revealed that the ncRNA-a recruits a transcription coactivator complex, Mediator, to facilitate chromosomal interaction between the ncRNA-a loci and its targets (Figure 2) (Lai et al., 2013). Mediator forms a complex with cohesin that creates a ring-like structure to keep two DNA segments together, which then regulates gene expression by connecting the enhancers and promoters of active genes in a cell-type specific manner (Kagey et al., 2010). In parallel, ncRNA-a stimulates the CDK8 kinase activity of Mediator to increase the level of histone H3 phosphorylation at serine 10 (H3S10), which is a mark associated with active chromatin and gene induction (Nowak and Corces, 2004).
Other lncRNAs also show related functions in different biological contexts. A Notch-regulated lncRNA, LUNAR1 (leukemia-induced noncoding activator RNA), enhances IGF1R mRNA expression by a mechanism similar to the ncRNA-a (Trimarchi et al., 2014). Importantly, the enhancer-like activity of LUNAR1 for IGF1R expression was critical for the growth of T cell acute lymphoblastic leukemia cells both in vitro and in vivo. HOTTIP is a lncRNA expressed from the tip of the HOXA locus that coordinates the activation of several HOXA genes in vivo (Wang et al., 2011). Knockdown of HOTTIP specifically decreases expression of distally located HOXA genes, but not the highly homologous HOXD genes, which suggests a cis mechanism. Unlike ncRNA-a, HOTTIP does not affect the chromosomal interaction. Instead, chromosomal looping brings HOTTIP into close proximity to the HOXA gene locus where HOTTIP promotes histone H3 lysine 4 trimethylation and gene transcription by recruiting WDR5/MLL methyltransferase complexes. Nest (nettoie Salmonella pas Theiler’s [cleanup Salmonella not Theiler’s]) is another enhancer-like lncRNA that works together with WDR5 to increase H3K4me3 level at the interferon-γ (Ifng) gene in activated T cells. Transgenic overexpression of NeST was shown to induce IFN-γ synthesis in activated CD8+ T cells, suggesting a possible trans-mechanism to regulate its neighboring gene. Interestingly, a recent study found the previously described lncRNA, ncRNA-a3 mapping to a bi-directionally transcribed enhancer of the TAL1 gene (Orom et al., 2010; Vucicevic et al., 2015). Therefore, it is likely that as eRNAs in different human cells are fully catalogued, many of the currently annotated lncRNAs with enhancer-like function will fall under the classification of eRNAs (Vucicevic et al., 2015).
Prospects
Transcription activity at enhancers was first hinted by the promoter-like activity (i.e., able to initiate transcription) of the first viral enhancer, the 72 bp tandem DNA repeats located upstream of SV40 early gene. Subsequently, several cellular LCRs and enhancers were also shown to transcribe ncRNAs. Nonetheless, transcriptional activity was not been regarded as a general feature of enhancers until the advent of genome-wide studies. It now seems clear that ncRNA transcription is a signature of functionally active enhancers at least in higher metazoans.
As described above, some experimental evidence already supoprts the roles of both enhancer transcription and the eRNA transcript in gene expression. However, we are still far from fully understanding the functional and biological significance of eRNAs, and more thorough studies on eRNA function and mechanism will be required. For example, the molecular determinants of eRNA function have not been studied, and thus it is not known if specific sequences or secondary structures would be critical for eRNA function. Moreover, although some studies found that only the sense eRNAs–transcribed in the same direction with its target mRNA–appear to be sufficient for the eRNA function (Lam et al., 2013; Li et al., 2013), it is not clear at this point whether strand-specific functionality is a general feature of eRNAs. It also needs to be mentioned that all current functional studies of eRNAs have relied exclusively on knockdown and/or overexpression approaches in cell culture, hence in vivo relevance is yet to be validated. While in vitro analytical methods offer technical advantages in mechanistic studies, several recent examples show that the findings from cell line studies in vitro are not observed or are quite different in knockout animals (Kohtz, 2014). Therefore determining the biological significance of eRNAs in an in vivo context is imperative.
The functionality question aside, widespread observation of transcribed enhancers across multiple mammalian cell types calls for revising the traditional definition of “promoters” as being the DNA regions that allow accurate transcription initiation of a gene. Similar to a promoter, an enhancer can direct RNA transcription from a defined site by independent RNAPII transcription machinery assembled with general TFs. Initiation of bidirectional transcription is another shared feature of transcriptional regulatory elements. Moreover, many of the features of upstream anti-sense transcripts mirror those of eRNAs, including their inherent instability and their enrichment for the tyrosine 1 phosphorylated form of RNAPII.
Importantly, the distinctive characteristic of the promoters is their ability to direct transcription of a spliced, polyadenylated transcript. In contrast to the promoter-driven mRNAs, eRNAs and upstream anti-sense RNAs are shorter in length (a few hundreds up to a few kilobases) and, by and large less stable. In addition, they are commonly subject to early termination through the action of the Integrator complex, which is consistent with their lack of 5′ splice sites and polyadenylation-dependent cleavage. However, as far as transcription initiation is concerned, there appears to be very little difference between the promoter and the enhancer. Indeed, in many examples, enhancers may look reminiscent of weak promoters transcribing low levels of RNAs. Additional studies will certainly be needed before we can fully understand and define the structural and functional identities of enhancers and promoters, and their interrelationship. Nonetheless, the recent unveiling of shared transcriptional architectures between the two regulatory domains compels us to revise our old ways of thinking and incorporate new models for transcriptional regulation in eukaryotes.
Acknowledgments
This work was supported by The Welch foundation (I-1786), The Klingenstein Fund, and NIH-NINDS R01NS085418 (T.-K.K). The work cited from Shiekhattar’s laboratory was supported by funds from Sylvester Comprehensive Cancer Center at University of Miami Miller School of Medicine and by grants R01 GM078455 and R01 GM105754 from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelman K, Lis JT. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet. 2012;13:720–731. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts S. Computational strategies for the genome-wide identification of cis-regulatory elements and transcriptional targets. Curr Top Dev Biol. 2012;98:121–145. doi: 10.1016/B978-0-12-386499-4.00005-7. [DOI] [PubMed] [Google Scholar]
- Akbari OS, Bae E, Johnsen H, Villaluz A, Wong D, Drewell RA. A novel promoter-tethering element regulates enhancer-driven gene expression at the bithorax complex in the Drosophila embryo. Development. 2008:123–131. doi: 10.1242/dev.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almada AE, Wu X, Kriz AJ, Burge CB, Sharp PA. Promoter directionality is controlled by U1 snRNP and polyadenylation signals. Nature. 2013;499:360–363. doi: 10.1038/nature12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R, Gebhard C, Miguel-Escalada I, Hoof I, Bornholdt J, Boyd M, Chen Y, Zhao X, Schmidl C, Suzuki T, et al. An atlas of active enhancers across human cell types and tissues. Nature. 2014;507:455–461. doi: 10.1038/nature12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner E, Daub CO, Vitting-Seerup K, Andersson R, Lilje B, Drablos F, Lennartsson A, Ronnerblad M, Hrydziuszko O, Vitezic M, et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science. 2015 doi: 10.1126/science.1259418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe HL, Monks J, Wijgerde M, Fraser P, Proudfoot NJ. Intergenic transcription and transinduction of the human beta-globin locus. Genes Dev. 1997;11:2494–2509. doi: 10.1101/gad.11.19.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison ML. Enhancers: mechanisms of action and cell specificity. Annu Rev Cell Biol. 1988;4:127–153. doi: 10.1146/annurev.cb.04.110188.001015. [DOI] [PubMed] [Google Scholar]
- Banerji J, Olson L, Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983;33:729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Banerji J, Rusconi S, Schaffner W. Expression of a β-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981;27:299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Barrera LO, Li Z, Smith AD, Arden KC, Cavenee WK, Zhang MQ, Green RD, Ren B. Genome-wide mapping and analysis of active promoters in mouse embryonic stem cells and adult organs. Genome Res. 2008;18:46–59. doi: 10.1101/gr.6654808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C, Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981;290:304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Berg MG, Singh LN, Younis I, Liu Q, Pinto AM, Kaida D, Zhang Z, Cho S, Sherrill-Mix S, Wan L, et al. U1 snRNP determines mRNA length and regulates isoform expression. Cell. 2012;150:53–64. doi: 10.1016/j.cell.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Bienz M, Pelham HR. Heat shock regulatory elements function as an inducible enhancer in the Xenopus hsp70 gene and when linked to a heterologous promoter. Cell. 1986;45:753–760. doi: 10.1016/0092-8674(86)90789-0. [DOI] [PubMed] [Google Scholar]
- Bohmann D, Keller W, Dale T, Scholer HR, Tebb G, Mattaj IW. A transcription factor which binds to the enhancers of SV40, immunoglobulin heavy chain and U2 snRNA genes. Nature. 1987;325:268–272. doi: 10.1038/325268a0. [DOI] [PubMed] [Google Scholar]
- Bonn S, Zinzen RP, Girardot C, Gustafson EH, Perez-Gonzalez A, Delhomme N, Ghavi-Helm Y, Wilczyński B, Riddell A, Furlong EEM. Tissue-specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nat Genet. 2012 doi: 10.1038/ng.1064. [DOI] [PubMed] [Google Scholar]
- Boyle AP, Davis S, Shulha HP, Meltzer P, Margulies EH, Weng Z, Furey TS, Crawford GE. High-resolution mapping and characterization of open chromatin across the genome. Cell. 2008;132:311–322. doi: 10.1016/j.cell.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky AS, Meyer CA, Swinburne IA, Hall G, Keenan BJ, Liu XS, Fox EA, Silver PA. Genomic mapping of RNA polymerase II reveals sites of co-transcriptional regulation in human cells. Genome Biol. 2005;6:R64. doi: 10.1186/gb-2005-6-8-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger M, Schubeler D, Bender MA, Hamilton J, Farrell CM, Hardison RC, Groudine M. A complex chromatin landscape revealed by patterns of nuclease sensitivity and histone modification within the mouse beta-globin locus. Mol Cell Biol. 2003;23:5234–5244. doi: 10.1128/MCB.23.15.5234-5244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- Cho H, Orphanides G, Sun X, Yang XJ, Ogryzko V, Lees E, Nakatani Y, Reinberg D. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol Cell Biol. 1998;18:5355–5363. doi: 10.1128/mcb.18.9.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium EP. Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Martins AL, Danko CG, Waters CT, Siepel A, Lis JT. Analysis of nascent RNA identifies a unified architecture of initiation regions at mammalian promoters and enhancers. Nat Genet. 2014;46:1311–1320. doi: 10.1038/ng.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–1848. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa F, Barozzi I, Mietton F, Ghisletti S, Polletti S, Tusi BK, Muller H, Ragoussis J, Wei CL, Natoli G. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010;8:e1000384. doi: 10.1371/journal.pbio.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descostes N, Heidemann M, Spinelli L, Schuller R, Maqbool MA, Fenouil R, Koch F, Innocenti C, Gut M, Gut I, et al. Tyrosine phosphorylation of RNA polymerase II CTD is associated with antisense promoter transcription and active enhancers in mammalian cells. Elife. 2014;3:e02105. doi: 10.7554/eLife.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, et al. Landscape of transcription in human cells. Nature. 2013;488:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttke SH, Lacadie SA, Ibrahim MM, Glass CK, Corcoran DL, Benner C, Heinz S, Kadonaga JT, Ohler U. Human Promoters Are Intrinsically Directional. Mol Cell. 2015 doi: 10.1016/j.molcel.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473:43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn RA, Almada AE, Zamudio JR, Sharp PA. Antisense RNA polymerase II divergent transcripts are P-TEFb dependent and substrates for the RNA exosome. Proc Natl Acad Sci U S A. 2011;108:10460–10465. doi: 10.1073/pnas.1106630108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M, Berg P. Deletion mapping of DNA regions required for SV40 early region promoter function in vivo. J Mol Appl Genet. 1982;1:457–481. [PubMed] [Google Scholar]
- Fromm M, Berg P. Simian virus 40 early- and late-region promoter functions are enhanced by the 72-base-pair repeat inserted at distant locations and inverted orientations. Mol Cell Biol. 1983;3:991–999. doi: 10.1128/mcb.3.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber M, Shilatifard A. Transcriptional elongation by RNA polymerase II and histone methylation. J Biol Chem. 2003;278:26303–26306. doi: 10.1074/jbc.R300014200. [DOI] [PubMed] [Google Scholar]
- Gillies SD, Morrison SL, Oi VT, Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983;33:717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Giresi PG, Kim J, McDaniell RM, Iyer VR, Lieb JD. FAIRE (Formaldehyde-Assisted Isolation of Regulatory Elements) isolates active regulatory elements from human chromatin. Genome Res. 2007;17:877–885. doi: 10.1101/gr.5533506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AD, Banaszynski LA, Noh KM, Lewis PW, Elsaesser SJ, Stadler S, Dewell S, Law M, Guo X, Li X, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140:678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribnau J, Diderich K, Pruzina S, Calzolari R, Fraser P. Intergenic transcription and developmental remodeling of chromatin subdomains in the human beta-globin locus. Mol Cell. 2000;5:377–386. doi: 10.1016/s1097-2765(00)80432-3. [DOI] [PubMed] [Google Scholar]
- Grosschedl R, Birnstiel ML. Spacer DNA sequences upstream of the T-A-T-A-A-A-T-A sequence are essential for promotion of H2A histone gene transcription in vivo. Proc Natl Acad Sci U S A. 1980;77:7102–7106. doi: 10.1073/pnas.77.12.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F, van Assendelft GB, Greaves DR, Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Guarente L. UASs and enhancers: common mechanism of transcriptional activation in yeast and mammals. Cell. 1988;52:303–305. doi: 10.1016/s0092-8674(88)80020-5. [DOI] [PubMed] [Google Scholar]
- Hah N, Benner C, Chong LW, Yu RT, Downes M, Evans RM. Inflammation-sensitive super enhancers form domains of coordinately regulated enhancer RNAs. Proc Natl Acad Sci U S A. 2015;112:E297–302. doi: 10.1073/pnas.1424028112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah N, Danko CG, Core L, Waterfall JJ, Siepel A, Lis JT, Kraus WL. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell. 2011;145:622–634. doi: 10.1016/j.cell.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah N, Murakami S, Nagari A, Danko CG, Kraus WL. Enhancer transcripts mark active estrogen receptor binding sites. Genome Res. 2013;23:1210–1223. doi: 10.1101/gr.152306.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- Henikoff S, Henikoff JG, Sakai A, Loeb GB, Ahmad K. Genome-wide profiling of salt fractions maps physical properties of chromatin. Genome Res. 2009;19:460–469. doi: 10.1101/gr.087619.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D, Schuijers J, Lin CY, Weintraub AS, Abraham BJ, Lee TI, Bradner JE, Young RA. Convergence of developmental and oncogenic signaling pathways at transcriptional super-enhancers. Mol Cell. 2015;58:362–370. doi: 10.1016/j.molcel.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y, Elefant F, Liebhaber SA, Cooke NE. Locus control region transcription plays an active role in long-range gene activation. Mol Cell. 2006;23:365–375. doi: 10.1016/j.molcel.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Hon G, Wang W, Ren B. Discovery and annotation of functional chromatin signatures in the human genome. PLoS Comput Biol. 2009;5:e1000566. doi: 10.1371/journal.pcbi.1000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Fei T, Chen Y, Li T, Gao Y, Wang X, Sun T, Sweeney CJ, Lee GS, Chen S, et al. Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proc Natl Acad Sci U S A. 2014;111:7319–7324. doi: 10.1073/pnas.1324151111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Zang C, Wei G, Cui K, Peng W, Zhao K, Felsenfeld G. H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat Genet. 2009;41:941–945. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Yu LR, Wang L, Zhang Z, Kasper LH, Lee JE, Wang C, Brindle PK, Dent SY, Ge K. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011;30:249–262. doi: 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida D, Berg MG, Younis I, Kasim M, Singh LN, Wan L, Dreyfuss G. U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature. 2010;468:664–668. doi: 10.1038/nature09479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikkonen MU, Spann NJ, Heinz S, Romanoski CE, Allison KA, Stender JD, Chun HB, Tough DF, Prinjha RK, Benner C, et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell. 2013;51:310–325. doi: 10.1016/j.molcel.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellis M, Wold B, Snyder MP, Bernstein BE, Kundaje A, Marinov GK, Ward LD, Birney E, Crawford GE, Dekker J, et al. Defining functional DNA elements in the human genome. Proc Natl Acad Sci U S A. 2014;111:6131–6138. doi: 10.1073/pnas.1318948111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G, Gruss P. Enhancer elements. Cell. 1983;33:313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F, Fenouil R, Gut M, Cauchy P, Albert TK, Zacarias-Cabeza J, Spicuglia S, de la Chapelle AL, Heidemann M, Hintermair C, et al. Transcription initiation platforms and GTF recruitment at tissue-specific enhancers and promoters. Nat Struct Mol Biol. 2011;18:956–963. doi: 10.1038/nsmb.2085. [DOI] [PubMed] [Google Scholar]
- Kohtz JD. Long non-coding RNAs learn the importance of being in vivo. Front Genet. 2014;5:45. doi: 10.3389/fgene.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S, Bohl D, Li C, Tuan D. Transcription of the HS2 enhancer toward a cis-linked gene is independent of the orientation, position, and distance of the enhancer relative to the gene. Mol Cell Biol. 1997;17:3955–3965. doi: 10.1128/mcb.17.7.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk MS, Hughes JR, Garrick D, Lynch MD, Sharpe JA, Sloane-Stanley JA, McGowan SJ, De Gobbi M, Hosseini M, Vernimmen D, et al. Intragenic Enhancers Act as Alternative Promoters. Mol Cell. 2012;45:447–458. doi: 10.1016/j.molcel.2011.12.021. [DOI] [PubMed] [Google Scholar]
- LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, Tollervey D. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell. 2005;121:713–724. doi: 10.1016/j.cell.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Lai F, Orom UA, Cesaroni M, Beringer M, Taatjes DJ, Blobel GA, Shiekhattar R. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501. doi: 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MT, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, Benner C, Kaikkonen MU, Kim AS, Kosaka M, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–515. doi: 10.1038/nature12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MT, Li W, Rosenfeld MG, Glass CK. Enhancer RNAs and regulated transcriptional programs. Trends Biochem Sci. 2014;39:170–182. doi: 10.1016/j.tibs.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings PP, Zhou Z, Vieira KF, Crusselle-Davis VJ, Bungert J. Recruitment of transcription complexes to the beta-globin locus control region and transcription of hypersensitive site 3 prior to erythroid differentiation of murine embryonic stem cells. FEBS J. 2006;273:746–755. doi: 10.1111/j.1742-4658.2005.05107.x. [DOI] [PubMed] [Google Scholar]
- Li G, Ruan X, Auerbach RK, Sandhu KS, Zheng M, Wang P, Poh HM, Goh Y, Lim J, Zhang J, et al. Extensive Promoter-Centered Chromatin Interactions Provide a Topological Basis for Transcription Regulation. Cell (Elsevier Inc) 2012:84–98. doi: 10.1016/j.cell.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X, et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498:516–520. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Ainol L, Zhang L, Yu X, Pi W, Tuan D. HS2 enhancer function is blocked by a transcriptional terminator inserted between the enhancer and the promoter. J Biol Chem. 2004;279:51704–51713. doi: 10.1074/jbc.M404039200. [DOI] [PubMed] [Google Scholar]
- Ling J, Baibakov B, Pi W, Emerson BM, Tuan D. The HS2 enhancer of the beta-globin locus control region initiates synthesis of non-coding, polyadenylated RNAs independent of a cis-linked globin promoter. J Mol Biol. 2005;350:883–896. doi: 10.1016/j.jmb.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Goodbourn S, Fischer JA. Regulation of inducible and tissue-specific gene expression. Science. 1987;236:1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Masternak K, Peyraud N, Krawczyk M, Barras E, Reith W. Chromatin remodeling and extragenic transcription at the MHC class II locus control region. Nat Immunol. 2003;4:132–137. doi: 10.1038/ni883. [DOI] [PubMed] [Google Scholar]
- May D, Blow MJ, Kaplan T, McCulley DJ, Jensen BC, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright C, et al. Large-scale discovery of enhancers from human heart tissue. Nat Genet. 2012;44:89–93. doi: 10.1038/ng.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, di Iulio J, Maleri S, Eser U, Vierstra J, Reynolds A, Sandstrom R, Stamatoyannopoulos JA, Churchman LS. Native elongating transcript sequencing reveals human transcriptional activity at nucleotide resolution. Cell. 2015;161:541–554. doi: 10.1016/j.cell.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo CA, Drost J, Wijchers PJ, van de Werken H, de Wit E, Vrielink JAFO, Elkon R, Melo SA, Léveillé N, Kalluri R, et al. eRNAs Are Required for p53-Dependent Enhancer Activity and Gene Transcription. Mol Cell. 2012:1–12. doi: 10.1016/j.molcel.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Montavon T, Soshnikova N, Mascrez B, Joye E, Thevenet L, Splinter E, De Laat W, Spitz F, Duboule D. A Regulatory Archipelago Controls Hox Genes Transcription in Digits. Cell (Elsevier Inc) 2011:1132–1145. doi: 10.1016/j.cell.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Moreau P, Hen R, Wasylyk B, Everett R, Gaub MP, Chambon P. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981;9:6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi K, Zare H, Dell’orso S, Grontved L, Gutierrez-Cruz G, Derfoul A, Hager GL, Sartorelli V. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol Cell. 2013;51:606–617. doi: 10.1016/j.molcel.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MM, Gerster T, Schaffner W. Enhancer sequences and the regulation of gene transcription. Eur J Biochem. 1988;176:485–495. doi: 10.1111/j.1432-1033.1988.tb14306.x. [DOI] [PubMed] [Google Scholar]
- Natoli G, Andrau JC. Noncoding Transcription at Enhancers: General Principles and Functional Models. Annu Rev Genet. 2011;46:120820103026000. doi: 10.1146/annurev-genet-110711-155459. [DOI] [PubMed] [Google Scholar]
- NE II, Heward JA, Roux B, Tsitsiou E, Fenwick PS, Lenzi L, Goodhead I, Hertz-Fowler C, Heger A, Hall N, et al. Long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat Commun. 2014;5:3979. doi: 10.1038/ncomms4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger MS. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J. 1983;2:1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak SJ, Corces VG. Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet. 2004;20:214–220. doi: 10.1016/j.tig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Ntini E, Jarvelin AI, Bornholdt J, Chen Y, Boyd M, Jorgensen M, Andersson R, Hoof I, Schein A, Andersen PR, et al. Polyadenylation site-induced decay of upstream transcripts enforces promoter directionality. Nat Struct Mol Biol. 2013;20:923–928. doi: 10.1038/nsmb.2640. [DOI] [PubMed] [Google Scholar]
- Orkin SH. Globin gene regulation and switching: circa 1990. Cell. 1990;63:665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Orom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parslow TG, Jones SD, Bond B, Yamamoto KR. The immunoglobulin octanucleotide: independent activity and selective interaction with enhancers. Science. 1987;235:1498–1501. doi: 10.1126/science.3029871. [DOI] [PubMed] [Google Scholar]
- Pekowska A, Benoukraf T, Zacarias-Cabeza J, Belhocine M, Koch F, Holota H, Imbert J, Andrau J-C, Ferrier P, Spicuglia S. H3K4 tri-methylation provides an epigenetic signature of active enhancers. EMBO J. 2011:4198–4210. doi: 10.1038/emboj.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preker P, Nielsen J, Kammler S, Lykke-Andersen S, Christensen MS, Mapendano CK, Schierup MH, Jensen TH. RNA exosome depletion reveals transcription upstream of active human promoters. Science. 2008;322:1851–1854. doi: 10.1126/science.1164096. [DOI] [PubMed] [Google Scholar]
- Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge SJ, Proudfoot NJ. Definition of transcriptional promoters in the human beta globin locus control region. J Mol Biol. 2002;323:601–611. doi: 10.1016/s0022-2836(02)01011-2. [DOI] [PubMed] [Google Scholar]
- Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2013;489:109–113. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaukowitch K, Joo JY, Liu X, Watts JK, Martinez C, Kim TK. Enhancer RNA facilitates NELF release from immediate early genes. Mol Cell. 2014;56:29–42. doi: 10.1016/j.molcel.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs BS, Gilchrist DA, Nechaev S, Muse GW, Burkholder A, Fargo DC, Adelman K. Bidirectional Transcription Arises from Two Distinct Hubs of Transcription Factor Binding and Active Chromatin. Mol Cell. 2015;58:1101–1112. doi: 10.1016/j.molcel.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seila AC, Calabrese JM, Levine SS, Yeo GW, Rahl PB, Flynn RA, Young RA, Sharp PA. Divergent transcription from active promoters. Science. 2008;322:1849–1851. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selth LA, Sigurdsson S, Svejstrup JQ. Transcript Elongation by RNA Polymerase II. Annu Rev Biochem. 2010;79:271–293. doi: 10.1146/annurev.biochem.78.062807.091425. [DOI] [PubMed] [Google Scholar]
- Serfling E, Lubbe A, Dorsch-Hasler K, Schaffner W. Metal-dependent SV40 viruses containing inducible enhancers from the upstream region of metallothionein genes. EMBO J. 1985;4:3851–3859. doi: 10.1002/j.1460-2075.1985.tb04157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Yue F, McCleary DF, Ye Z, Edsall L, Kuan S, Wagner U, Dixon J, Lee L, Lobanenkov VV, et al. A map of the cis-regulatory sequences in the mouse genome. Nature. 2012 doi: 10.1038/nature11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale ST, Kadonaga JT. The RNA polymerase II core promoter. Annu Rev Biochem. 2003;72:449–479. doi: 10.1146/annurev.biochem.72.121801.161520. [DOI] [PubMed] [Google Scholar]
- Struhl K. Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat Struct Mol Biol. 2007;14:103–105. doi: 10.1038/nsmb0207-103. [DOI] [PubMed] [Google Scholar]
- Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, Sheffield NC, Stergachis AB, Wang H, Vernot B, et al. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie F, Banerjee R, Stratton CA, Prasad-Sinha J, Stepanik V, Zlobin A, Diaz MO, Scacheri PC, Harte PJ. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development. 2009;136:3131–3141. doi: 10.1242/dev.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. Chromatin modification by DNA tracking. Proc Natl Acad Sci U S A. 1999;96:13634–13637. doi: 10.1073/pnas.96.24.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi T, Bilal E, Ntziachristos P, Fabbri G, Dalla-Favera R, Tsirigos A, Aifantis I. Genome-wide Mapping and Characterization of Notch-Regulated Long Noncoding RNAs in Acute Leukemia. Cell. 2014;158:593–606. doi: 10.1016/j.cell.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D, Kong S, Hu K. Transcription of the hypersensitive site HS2 enhancer in erythroid cells. Proc Natl Acad Sci USA. 1992;89:11219–11223. doi: 10.1073/pnas.89.23.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernimmen D, De Gobbi M, Sloane-Stanley JA, Wood WG, Higgs DR. Long-range chromosomal interactions regulate the timing of the transition between poised and active gene expression. EMBO J. 2007;26:2041–2051. doi: 10.1038/sj.emboj.7601654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira KF, Levings PP, Hill MA, Crusselle VJ, Kang SHL, Engel JD, Bungert J. Recruitment of transcription complexes to the beta-globin gene locus in vivo and in vitro. J Biol Chem. 2004;279:50350–50357. doi: 10.1074/jbc.M408883200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright C, Chen F, et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;457:854–858. doi: 10.1038/nature07730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucicevic D, Corradin O, Ntini E, Scacheri PC, Orom UA. Long ncRNA expression associates with tissue-specific enhancers. Cell Cycle. 2015;14:253–260. doi: 10.4161/15384101.2014.977641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta RA, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Carroll JS, Brown M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell. 2005;19:631–642. doi: 10.1016/j.molcel.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Weingarten-Gabbay S, Segal E. A shared architecture for promoters and enhancers. Nat Genet. 2014;46:1253–1254. doi: 10.1038/ng.3152. [DOI] [PubMed] [Google Scholar]
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyers F, Rougemaille M, Badis G, Rousselle JC, Dufour ME, Boulay J, Regnault B, Devaux F, Namane A, Seraphin B, et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell. 2005;121:725–737. doi: 10.1016/j.cell.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Yoo EJ, Cooke NE, Liebhaber SA. An RNA-independent linkage of noncoding transcription to long-range enhancer function. Mol Cell Biol. 2012;32:2020–2029. doi: 10.1128/MCB.06650-11. [DOI] [PMC free article] [PubMed] [Google Scholar]