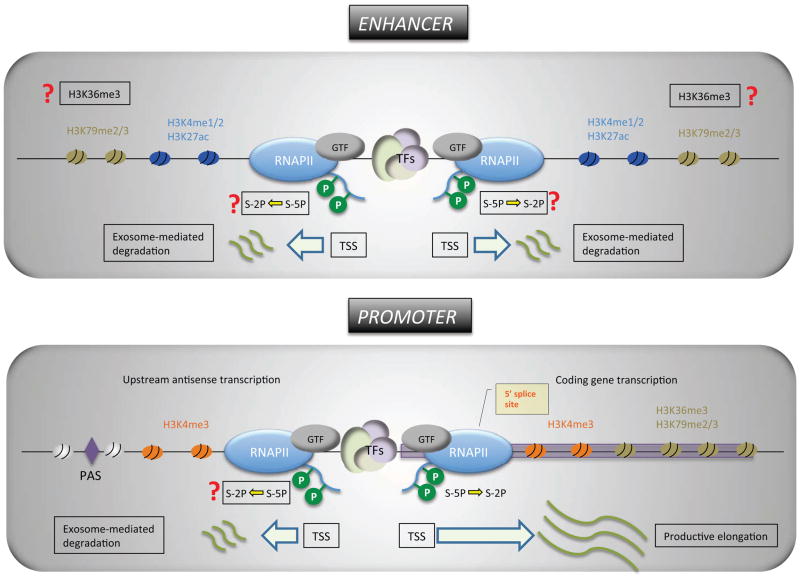
Figure 1. A contemporary view on promoters and enhancers.
Features of promoters include: Transcription initiation in the sense and anti-sense direction is mediated by the transcription machinery assembled independently onto its own core promoter. Although not shown here, convergent transcription has been observed at the promoters of weakly expressed genes. H3K4me3 is highly enriched at the promoter regions. Enhancer-like chromatin signatures (H3K4me1 and H3K27ac) and the Tyr-1P form of the RNAPII have also been observed near the upstream anti-sense TSSs. Polyadenylation sites are enriched near the 3′ end of the upstream anti-sense RNAs and mediate the exosome-dependent degradation of the antisense RNAs. 5′ splice sites are only present in the coding gene, and might contribute to the productive elongation of sense mRNA transcripts through the binding of the U1 splicing complex, which blocks PAS-mediated early termination. The Ser-5P form of RNAPII is engaged in upstream anti-sense transcription, but it is not known if Ser-2P of RNAPII occurs during the elongation of anti-sense RNA.
Features of enhancers include: Like the promoter, the enhancer recruits the general transcription factors (GTF) including RNAPII and initiates transcription at defined sites. Enhancer-driven transcription typically exhibits more prominent bi-directionality than that stemming from the promoter. H3K4me1/2 is commonly enriched at enhancers. Functionally active enhancers also exhibit a high level of H3K27 acetylation whereas poised or inactive enhancers are marked by H3K27me3. Ser-5P and Tyr-1P forms of the RNAPII have been observed. It is not clear whether or not Ser-2P RNAPII and H3K36me3 marks are present at active enhancers. 5′ splice site sequences are not enriched near the regions surrounding enhancers. Both strands of enhancer RNAs appear to be degraded by the exosome, although it is not known whether it is mediated by the PAS-dependent mechanism.