Abstract
Purpose
We assess the impact of obesity, as measured conventionally by body mass index vs excess adiposity as measured by fat mass index, on mortality after radical cystectomy for bladder cancer, adjusting for the presence of skeletal muscle wasting.
Materials and Methods
This retrospective cohort study included 262 patients treated with radical cystectomy for bladder cancer between 2000 and 2008 at the Mayo Clinic. Lumbar skeletal muscle and adipose compartment areas were measured on preoperative imaging. Overall survival was compared according to gender specific consensus fat mass index and skeletal muscle index thresholds as well as conventional body mass index based criteria. Predictors of all cause mortality were assessed by multivariable modeling.
Results
Increasing body mass index correlated with improved overall survival (p=0.03) while fat mass index based obesity did not (p=0.08). After stratification by sarcopenia, no obesity related 5-year overall survival benefit was observed (68% vs 51.4%, p=0.2 obese vs normal and 40% vs 37.4%, p=0.7 sarcopenia vs sarcopenic/obese). On multivariable analysis class I obesity according to body mass index (HR 0.79, p=0.33) or fat mass index criteria (HR 0.85, p=0.45) was not independently associated with all cause mortality after adjusting for sarcopenia (HR 1.7, p=0.01) as well as age, performance status, pTN stage and smoking status. However, in patients with normal lean muscle mass each 1 kg/m2 increase in weight or adipose mass was associated with a 7% to 14% decrease in all cause mortality.
Conclusions
After adjusting for lean muscle wasting, neither measurements of obesity nor adiposity were significantly associated with all cause mortality in patients treated with radical cystectomy, although subanalyses suggest a potential benefit among those with normal lean muscle mass.
Keywords: sarcopenia, obesity, mortality, cystectomy, body mass index
Worldwide approximately 1.1 billion adults are estimated to be overweight and an additional 475 million are obese. Despite the substantial adverse health outcomes associated with obesity,1 there is a growing body of literature suggesting that overweight and obese surgical patients have decreased all cause mortality compared to leaner patients.2 This counterintuitive finding has been coined “the obesity paradox”3,4 but has yet to be described among patients treated with radical cystectomy, in whom the impact of obesity remains unclear.5–8
It was estimated that in the United States approximately 75,000 patients would be diagnosed with urothelial carcinoma of the bladder in 2014,9 of whom approximately 75% were overweight or obese,6 and for whom the standard of care is neoadjuvant chemotherapy followed by radical cystectomy with bilateral pelvic lymph node dissection and urinary diversion. Unfortunately, even in contemporary series the combined surgical and disease morbidity results in 5-year overall survival rates of only 42% to 58%.10,11
In a recent study of a contemporary radical cystectomy cohort we observed a significant risk of increased mortality in patients with sarcopenia, or severe skeletal muscle wasting.12 We also observed an inverse trend, consistent with the obesity paradox, toward decreased mortality with increasing BMI on unadjusted analysis. However, after adjusting for tumor specific factors, comorbidity and skeletal muscle wasting, BMI was no longer independently associated with all cause mortality.
We hypothesize that our previous findings were related to the nonspecificity of BMI as a measure of body composition, such that patients with a low BMI were likely those who also had skeletal muscle wasting, which strongly predicts poor outcomes in patients with cancer.12 Thus, we explored the association between obesity as classified by BMI vs FMI, a measure of adiposity and overall survival after RC, adjusting for the presence of lean muscle wasting.
Methods
Patient Selection
After institutional review board approval we retrospectively identified 515 consecutive patients treated with RC and urinary diversion between 2000 and 2008. Patients were excluded from analysis if preoperative digital imaging was not available within 30 days of surgery (244) or if image analysis was precluded by poor image quality (9), leaving 262 patients (225 men and 37 women) available for analysis.13,14
Body Composition Analysis
A representative axial image at the level of L3 was identified by one of 2 radiologists (GDS, MRM). Then cross-sectional skeletal muscle and adipose areas were measured according to attenuation thresholds using SliceOmatic software (v.5.0, Tomovision, Quebec, Canada) by 1 investigator (SPP) who was blinded to patient outcome. Skeletal muscle area was identified using an attenuation threshold of −29 to +150 HU.12,15 SMI was calculated by normalizing the total skeletal muscle area by height squared (cm2/m2). Patients were classified as sarcopenic according to gender specific international consensus reference values, which represent muscularity below the fifth percentile for healthy young adults (male—SMI less than 55 cm2/m2, female—SMI less than 39 cm2/m2).14
Total adipose tissue area includes the total cross-sectional area of all visceral (−150 to −50 HU), intramuscular and subcutaneous adipose tissue (−190 to −30 HU, cm2) on the L3 axial CT image. Whole body FM (kg) was then calculated using the equation,
FMI was calculated by normalizing FM (kg) by height squared (m2). Patients were classified as obese if they met the NHANES 2009 criteria for class I obesity (male—FMI greater than 9 kg/m2 , female—FMI greater than 13 kg/m2).17 Table 1 shows a comparison of FMI and BMI based criteria for obesity. Agreement of obesity classification was assessed using the kappa statistic for obesity as categorized by BMI vs FMI.
Table 1. Obesity classification criteria according to WHO classification of obesity by BMI and FMI generated from the NHANES cohort.
| Category | BMI (kg/m2) | Category | Male FMI (kg/m2) | Female FMI (kg/m2) |
|---|---|---|---|---|
| Underweight | Less than 18.5 | Severe fat deficit, moderate fat deficit, mild fat deficit, normal | Less than 2, 2–less than 2.3, 2.3–less than 3, 3–6 | Less than 3.5, 3.5–less than 4, 4–less than 5, 5–9 |
| Normal | 18.5–24.9 | |||
| Overweight (pre-obese) | 25–29.9 | Excess fat | Greater than 6–9 | Greater than 9–13 |
| Obese: | 30.0 or Greater | Obese: | Greater than 9 | Greater than 13 |
| Class I (mild obesity) | 30.0–34.9 | Class I | Greater than 9–12 | Greater than 13–17 |
| Class II (moderate obesity) | 35–39.9 | Class II | Greater than 12–15 | Greater than 17–21 |
| Class III (morbid obesity) | 40.0 or Greater | Class III | Greater than 15 | Greater than 21 |
Patients were then grouped according to combined SMI and FMI classifications of low SMI and high FMI (sarcopenic obese), normal SMI and high FMI (obese), normal SMI and FMI (normal), and low SMI and normal FMI (sarcopenic). Representative axial CT for patients in each group is shown in figure 1.
Figure 1.
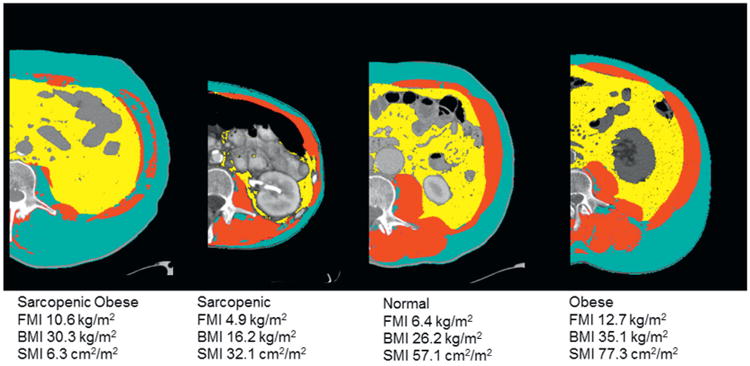
Representative images for each cohort of body composition demonstrating differences in skeletal muscle area (red), and visceral (yellow) and subcutaneous/intramuscular adipose areas (teal) among sarcopenic, normal, obese and sarcopenic obese individuals.
Statistical Analysis
We compared clinicopathological variables across the 4 groups. Continuous features were summarized with means (SD) or medians (IQR) as appropriate and compared using the t-test or Wilcoxon rank sum test. Categorical features were summarized with frequency counts (percentages), and compared using the chi-square and Cochran-Armitage trend tests.
The primary outcome of interest was overall survival, defined as the total length of time between the dates of RC and patient death, for which time and cause were verified via death certificate. OS was compared between BMI and FMI strata, as well as among the 4 patient groups defined by FMI/SMI using the log rank test.
Associations with death from any cause were evaluated using univariable and multivariable Cox proportional hazards regression models. Two multivariable models were created, incorporating obesity as classified by BMI or FMI. A subanalysis was performed assessing the impact of obesity on nonsarcopenic patients. C-statistics were calculated for each model. Statistical analyses were performed using the SAS® software package. All tests were 2-sided and p <0.05 was considered statistically significant.
Results
To test for possible bias introduced by the exclusion of patients with missing digital imaging, clinicopathological features and the survival of patients with missing data were compared to patients included in the analytic cohort, demonstrating no significant differences (data not shown). The distribution of obesity classification as defined by BMI or FMI based criteria is presented in figure 2, A, demonstrating poor agreement between these measures of obesity (kappa statistic 0.41, 95% CI 0.33–0.49, p <0.0001).
Figure 2.
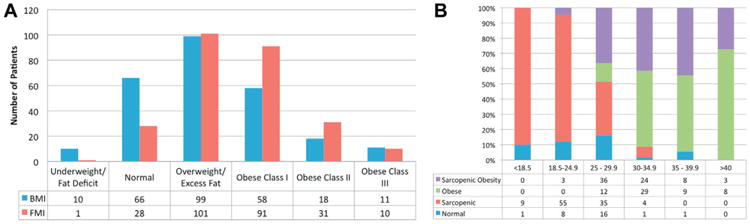
A, comparison of study cohort distribution according to WHO classification of obesity by BMI to classification ranges for FMI generated from NHANES cohort (kappa 0.412, 95% CI 0.33–0.49, p <0.0001). B, percentage of patients (262) according to body composition type of sarcopenic, sarcopenic obese, normal and obese per BMI strata (kg/m2).
Figure 2, B shows the distribution of patients according to sarcopenia and class I FMI based obesity compared to categorization by traditional BMI criteria. For example, a patient with normal muscularity by SMI criteria and normal adiposity by FMI criteria (27) may have a BMI that ranges anywhere from 18.5 to 39.9 kg/m2. Conversely, only 12% (8 of 66) of the patients with a normal BMI had a normal SMI and FMI, whereas 83% (55 of 66) met the criteria for sarcopenia and 4.5% (3 of 66) were sarcopenic obese.
Clinicopathological features of the 262 patients are presented in supplementary table 1 (http://jurology.com/), stratified by body composition. Patients with sarcopenia were significantly older than nonsarcopenic patients regardless of obesity status (p=0.01). The highest rates of current/prior smoking were noted in sarcopenic obese patients (90.3% vs normal 61.5%, sarcopenic 81.2% and obese 78.9%, p=0.01). Otherwise there were no significant differences among the patient groups.
Median anthropomorphic measurements are presented in supplementary table 2 (http://jurology.com/). Overall 67.6% (177) of patients were sarcopenic. Median BMI was 27.7 kg/m2. Median FMI ranged from 7.7 to 8.0 kg/m2 in normal and sarcopenic patients to 10.6 to 11.6 kg/m2 in obese and sarcopenic obese patients.
At last followup 174 (66.4%) patients had died, of whom 116 (44.2%) died of bladder cancer. Median followup was 6.3 years (IQR 5.7-9.5). Overall survival demonstrated an inverse, stepwise association with BMI strata (fig. 3, A), such that 5-year OS was 30%, 41.4%, 46% and 54.1% for patients with BMI less than 18.5, 18.5 to 24.9, 25 to 29.9 and 30 or greater kg/m2, respectively (p=0.03). A similar trend was noted when comparing strata of adiposity with 5-year OS of 35%, 45% and 51% for normal/fat deficit, excess fat and obese, respectively (p=0.08; fig. 3, B), although this did not achieve statistical significance.
Figure 3.
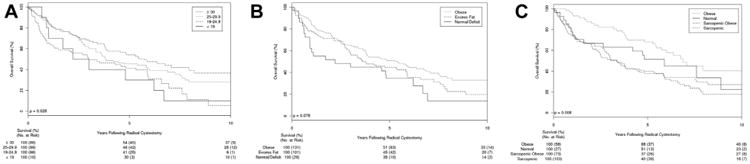
A, OS according to WHO defined BMI categories. B, OS according to FMI categories. Patients with fat deficit were combined into normal adipose tissue category due to low number (3). C, OS according to body composition according to obesity status by FMI based criteria and presence or absence of sarcopenia. OS was similar between nonsarcopenic patients with and without obesity (p = 0.17), and between sarcopenic obese and sarcopenic patients (p = 0.72).
OS was also compared between FMI obese and nonobese patients, stratified by sarcopenia (fig. 3, C). Among nonsarcopenic patients those who were obese and those who were nonobese had a similar 5-year OS (68% vs 51%, p=0.2). Similarly, among sarcopenic patients the 5-year OS was similar for those with obesity and those without (40% vs 37.4%, p=0.72). However, overall sarcopenic patients had a shorter OS than those without sarcopenia, regardless of obesity (p=0.008 overall).
Table 2 depicts univariable associations between clinicopathological features and all cause mortality. There was no significant association between BMI based obesity classification and ACM (HR 0.68, p=0.14), while FMI obesity was associated with a trend toward increased ACM (HR 0.75, p=0.06). As a continuous variable FMI was associated with a 7% decrease in the risk of ACM per unit increase in kg/m2 (HR 0.93, p=0.008) and BMI was associated with a 4% decrease in the risk of ACM per unit increase in kg/m2 (HR 0.96, p=0.005).
Table 2. Univariable associations with ACM.
| HR | 95% CI | p Value | |
|---|---|---|---|
| Pt features: | |||
| Age at surgery* | 1.03 | 1.01–1.05 | 0.0002 |
| Smoking status (Ref = no/never) | 1.51 | 1.00–2.3 | 0.05 |
| Gender (Ref = female) | 1.17 | 0.76–1.79 | 0.49 |
| ASA score (Ref = ASA 1-2) | 1.90 | 1.38–2.6 | <0.0001 |
| ECOG* | 1.33 | 1.10–1.60 | 0.002 |
| Pathological features: | |||
| Tumor stage at surgery (Ref = pT2 or less) | 2.36 | 1.75–3.19 | <0.0001 |
| Nodal stage at surgery (Ref = N0/X) | 1.90 | 1.38–2.60 | <0.0001 |
| Continuous body composition features:* | |||
| FMI (kg/m2) | 0.93 | 0.87–0.98 | 0.008 |
| BMI (kg/m2) | 0.96 | 0.94–0.99 | 0.005 |
| SMI (cm2/m2) | 0.98 | 0.96–0.99 | 0.003 |
| Skeletal muscle attenuation | 0.99 | 0.98–1.00 | 0.12 |
| Categorical body composition features: | |||
| Obese class I/II/III (Ref = normal/excess fat) | 0.75 | 0.56–1.01 | 0.06 |
| Obese (Ref = BMI less than 30 kg/m2) | 0.68 | 0.53–1.09 | 0.14 |
| Sarcopenia (Ref = no) | 1.75 | 1.24–2.50 | 0.001 |
| 4-Level body composition: | |||
| Normal | Ref | Ref | Ref |
| Sarcopenic | 1.40 | 0.82–2.41 | 0.22 |
| Obese | 0.69 | 0.38–1.28 | 0.24 |
| Sarcopenic obesity | 1.29 | 0.74–2.27 | 0.40 |
Continuous variable: represents the hazard ratio for an increase of 1 unit of the variable being tested.
In multivariable models adjusted for age, smoking status, ASA® and ECOG score, and pTN stage, neither obesity as categorized by BMI (HR 0.79, p=0.33) nor FMI (HR 0.85, p=0.45) criteria was independently associated with ACM, while sarcopenia was independently associated with a 67% to 71% increased risk of ACM (HR 1.67−1.71, p=0.01, c-index 0.73, table 3).
Table 3. Multivariable associations with ACM comparing models using obesity as classified by BMI or FMI.
| HR (95% CI) | p Value | |
|---|---|---|
| Model 1 BMI: | ||
| Age at surgery* | 1.03 (1.01–1.05) | 0.006 |
| Smoking status (Ref = no/never) | 1.25 (0.81–1.94) | 0.31 |
| ASA score (Ref = ASA 1-2) | 1.82 (1.27–2.62) | 0.001 |
| ECOG* | 1.14 (0.93–1.41) | 0.21 |
| Tumor stage at surgery (Ref = pT2 or less) | 2.33 (1.69–3.20) | <0.0001 |
| Nodal stage at surgery (Ref = N0/X) | 1.58 (1.12–2.23) | 0.009 |
| Sarcopenia (Ref = no) | 1.71 (1.14–2.57) | 0.01 |
| Class I obesity or greater (BMI criteria, Ref = no) | 0.79 (0.50–1.26) | 0.33 |
| c-index 0.73 | ||
| Model 2 FMI: | ||
| Age at surgery* | 1.03 (1.01–1.05) | 0.002 |
| Smoking status (Ref = no/never) | 1.24 (0.81–1.92) | 0.33 |
| ASA score (Ref = ASA 1-2) | 1.80 (1.26–2.58) | 0.001 |
| ECOG* | 1.15 (0.931–141) | 0.19 |
| Tumor stage at surgery (Ref = pT2 or less) | 2.33 (1.70–3.21) | <0.0001 |
| Nodal stage at surgery (Ref = N0/X) | 1.59 (1.13–2.24) | 0.008 |
| Sarcopenia (Ref = no) | 1.67 (1.11–2.50) | 0.01 |
| Class I obesity or greater (FMI criteria, Ref = no) | 0.85 (0.56–1.29) | 0.45 |
| c-index 0.73 |
Similar models incorporating BMI and FMI as continuous variables demonstrated no independent relationship between increases in BMI (HR 0.98, p=0.1, c-index 0.7) or FMI (HR 0.94, p=0.08, c-index 0.7).
Continuous variable: represents the change in hazard ratio for an increase of 1 unit of the variable being tested.
However, a subanalysis of nonsarcopenic patients demonstrated a trend toward a decreased risk of ACM associated with obesity by FMI criteria (HR 0.53, 95% CI 0.28−1.0, p=0.05) and by BMI criteria (HR 0.58, 95% CI 0.32−1.07, p=0.08) after adjustment for age, ASA score and tumor stage (supplementary table 3, http://jurology.com/). When these features were assessed as continuous variables, we observed a 7% decrease in the risk of ACM per unit increase of 1 kg/m2 in BMI and a 14% decrease in the risk of ACM per 1 kg/m2 increase in FMI.
Discussion
In this study we observed that the determination of obesity by BMI resulted in misclassification of body composition compared to assessments of skeletal muscle and adipose compartments by SMI and FMI derived from standard preoperative cross-sectional imaging. As highlighted by our data, weight normalized by height inadequately portrays excess lean muscularity or adiposity. Simply put, BMI measures excess weight but not excess fat.
Consistent with the reported obesity paradox,18,19 on unadjusted analysis we observed an inverse stepwise association between strata of BMI or FMI and mortality. However, on adjusted analysis, variation in lean muscularity attenuated this relationship. Essentially, in the setting of skeletal muscle wasting (sarcopenia), there does not appear to be any benefit or additive harm specifically associated with excess adipose tissue, although interestingly, in the subanalysis of nonsarcopenic patients, multivariable analyses demonstrated an association between decreasing ACM with increasing BMI and FMI. To our knowledge, this study is the first to specifically assess how obesity impacts survival after radical cystectomy relative to lean muscularity and adiposity and to describe the obesity paradox in this population.
Among patients with urothelial carcinoma, prior reports have conflicted regarding the impact of obesity on post-RC survival. Chromecki et al observed that obesity (BMI greater than 30 kg/m2) is associated with increased recurrence, cancer specific mortality and ACM,6 while others reported no association between obesity and ACM20 or improved survival in obese patients.8 Additionally, obese patients have been reported to have lower rates of perioperative complications and surgical costs than normal weight patients, an observation in accord with the obesity paradox.21 Of note, in further analysis in the current cohort we observed that neither obesity by BMI nor FMI criteria was independently associated with cancer specific mortality on multivariate analysis accounting for sarcopenia (data not shown).
The observation that excess fat mass is not protective after controlling for skeletal muscle wasting has been observed in patients undergoing chemotherapy.22 Similarly, Prado et al reported that sarcopenia independently predicted survival in obese patients with solid organ tumors involving the respiratory and gastrointestinal tracts.23 These results and the findings from the current study suggest that the presence of sarcopenia undermines any apparent benefit noted with obesity, such that any protection afforded by fat mass occurs only in the setting of normal lean muscle mass, as demonstrated in the subanalysis of nonsarcopenic patients. The mechanism by which excess fat or excess weight is associated with improved survival in the setting of normal lean muscle mass remains to be established, but may be related to additional gains in lean muscularity or nutritional reserve.
It is important to recognize the possibility of occult skeletal muscle wasting in the setting of obesity. Axial CT images routinely obtained as part of preoperative staging offer the opportunity to define skeletal muscle and adipose tissue compartments, permitting detailed characterization of a patient's body composition.12,13,24 Regional analysis of lean and adipose tissue on axial CT at the level of L3, as performed here, has been validated in patients with cancer against dual energy x-ray absorptiometry scans, outperforming bioelectrical impedance analysis in terms of sensitivity and specificity.16
Limitations of this study include selection bias inherent to the retrospective study design, which also precluded our ability to correlate measurements of skeletal muscle wasting or obesity with metrics of nutritional status such as albumin,25,26 or functional measures of frailty such as handgrip strength or walking speed. Additionally, digital CT was unavailable for 244 patients treated with RC at our institution during the study period, as our institution is a referral center, thus preoperative imaging was frequently obtained at the patient's local or referring institution and not uniformly digitized. The effect of possible bias introduced by these missing data was addressed by comparing the characteristics of patients without imaging to those included in the study. Our analytic cohort was similar to patients with missing digital scans with respect to all clinicopathological features including BMI, urothelial cancer stage and grade, comorbidity, performance status and overall survival. Finally, given the trend toward a significant association between BMI and FMI as continuous variables on multivariate analysis, it is possible that a larger study may demonstrate that obesity is an independent predictor of overall survival in addition to sarcopenia.
Despite these limitations, to our knowledge the current study is the first to describe the impact of misclassification of body composition using BMI based definitions in a surgical cohort. The results of this study suggest that the obesity paradox, as described on the basis of BMI, may relate to weight differences due to skeletal muscle wasting rather than differences in adipose burden. The implications of this finding are that encouragement of weight gain alone through increased caloric intake in cachectic patients with cancer is unlikely to be successful in improving outcomes. Rather, interventions might focus on restoring skeletal muscle mass as well as physical robustness. In addition, these data highlight the importance of assessing obese patients for the occult presence of skeletal muscle wasting. Given that sarcopenia is increasingly acknowledged as a risk factor in the patient undergoing radical cystectomy12,27,28 and with the increasing use of neoadjuvant chemotherapy before surgery, which in and of itself may contribute to muscle wasting,29 the identification of patients with lean muscle wasting at diagnosis may provide a window of opportunity to offer these patients interventions to optimize them for surgery and preempt further muscle wasting.30 Although SMI and FMI are not yet readily measurable in daily clinical practice, the development of automatic algorithms to calculate these measurements from standard CT imaging is under way. This will allow urologists to incorporate these novel body composition measurements into clinical risk stratification and to potentially select patients who would benefit from preoperative intervention.
Conclusions
Precise anthropomorphic measurement of body composition quantifying adiposity and lean muscle mass demonstrates the lack of specificity of BMI in conveying body composition, and that obesity by conventional BMI or by FMI based criteria is not independently associated with overall survival after radical cystectomy. However, among patients with normal muscularity there is a trend toward improved survival in those with increasing weight and adiposity, consistent with the obesity paradox. Further study of the interaction between lean muscle mass and adipose burden is necessary to validate these findings, which may represent a novel and quantitative tool for preoperative risk stratification.
Supplementary Material
Acknowledgments
Supported by Grant UL1 TR000135 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations and Acronyms
- ACM
all cause mortality
- BMI
body mass index
- CT
computerized tomography
- ECOG
Eastern Cooperative Oncology Group
- FM
fat mass
- FMI
fat mass index
- NHANES
National Health and Nutrition Examination Survey
- OS
overall survival
- RC
radical cystectomy
- SMI
skeletal muscle index
Footnotes
Study received institutional review board approval.
Nothing to disclose.
References
- 1.James PT, Rigby N, Leach R, et al. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Prev Rehabil. 2004;11:3. doi: 10.1097/01.hjr.0000114707.27531.48. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giles KA, Hamdan AD, Pomposelli FB, et al. Body mass index: surgical site infections and mortality after lower extremity bypass from the National Surgical Quality Improvement Program 2005-2007. Ann Vasc Surg. 2010;24:48. doi: 10.1016/j.avsg.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullen JT, Davenport DL, Hutter MM, et al. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol. 2008;15:2164. doi: 10.1245/s10434-008-9990-2. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Chromecki TF, Cha EK, Fajkovic H, et al. Obesity is associated with worse oncological outcomes in patients treated with radical cystectomy. BJU Int. 2013;111:249. doi: 10.1111/j.1464-410X.2012.11322.x. [DOI] [PubMed] [Google Scholar]
- 7.Hafron J, Mitra N, Dalbagni G, et al. Does body mass index affect survival of patients undergoing radical or partial cystectomy for bladder cancer? J Urol. 2005;173:1513. doi: 10.1097/01.ju.0000154352.54965.14. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal G, Espiritu P, Luchey A, et al. Body-mass index is an independent predictor of survival in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. J Urol, suppl. 2014;141:e539. abstract PD18-12. [Google Scholar]
- 9.American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 10.Ploussard G, Shariat SF, Dragomir A, et al. Conditional survival after radical cystectomy for bladder cancer: evidence for a patient changing risk profile over time. Eur Urol. 2014;66:361. doi: 10.1016/j.eururo.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 11.Shariat SF, Karakiewicz PI, Palapattu GS, et al. Nomograms provide improved accuracy for predicting survival after radical cystectomy. Clin Cancer Res. 2006;12:6663. doi: 10.1158/1078-0432.CCR-06-0372. [DOI] [PubMed] [Google Scholar]
- 12.Psutka SP, Carrasco A, Schmidt GD, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer. 2014;120:2910. doi: 10.1002/cncr.28798. [DOI] [PubMed] [Google Scholar]
- 13.Martin L, Birdsell L, MacDonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 14.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 15.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 16.Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 17.Kelly TL, Wilson KE, Heymsfield SB. Dual energy x-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amundson DE, Djurkovic S, Matwiyoff GN. The obesity paradox. Crit Care Clin. 2010;26:583. doi: 10.1016/j.ccc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Valentijn TM, Galal W, Tjeertes EK, et al. The obesity paradox in the surgical population. Surgeon. 2013;11:169. doi: 10.1016/j.surge.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Bachir BG, Aprikian AG, Izawa JI, et al. Effect of body mass index on the outcomes of patients with upper and lower urinary tract cancers treated by radical surgery: results from a Canadian multicenter collaboration. Urol Oncol. 2014;32:441. doi: 10.1016/j.urolonc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Bagrodia A, Grover S, Srivastava A, et al. Impact of body mass index on clinical and cost outcomes after radical cystectomy. BJU Int. 2009;104:326. doi: 10.1111/j.1464-410X.2009.08358.x. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez MC, Pastore CA, Orlandi SP, et al. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 2014;99:999. doi: 10.3945/ajcn.113.071399. [DOI] [PubMed] [Google Scholar]
- 23.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarco-penic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 24.Prado CM, Wells JC, Smith SR, et al. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31:583. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasinghe PC, Ediriweera DS, Kumarage SK, et al. Pre-operative hypoalbuminaemia predicts poor overall survival in rectal cancer: a retrospective cohort analysis. BMC Clin Pathol. 2013;13:12. doi: 10.1186/1472-6890-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan TM, Tang D, Stratton KL, et al. Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol. 2011;59:923. doi: 10.1016/j.eururo.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AB, Deal AM, Yu H, et al. Sarcopenia as a predictor for complications and survival following radical cystectomy. J Urol. 2014;191:1714. doi: 10.1016/j.juro.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 28.Wan F, Zhu Y, Gu C, et al. Lower skeletal muscle index and early complications in patients undergoing radical cystectomy for bladder cancer. World J Surg Oncol. 2014;12:14. doi: 10.1186/1477-7819-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yip C, Goh V, Davies A, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 2014;24:998. doi: 10.1007/s00330-014-3110-4. [DOI] [PubMed] [Google Scholar]
- 30.Bibas L, Levi M, Bendayan M, et al. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134. doi: 10.1016/j.pcad.2014.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


