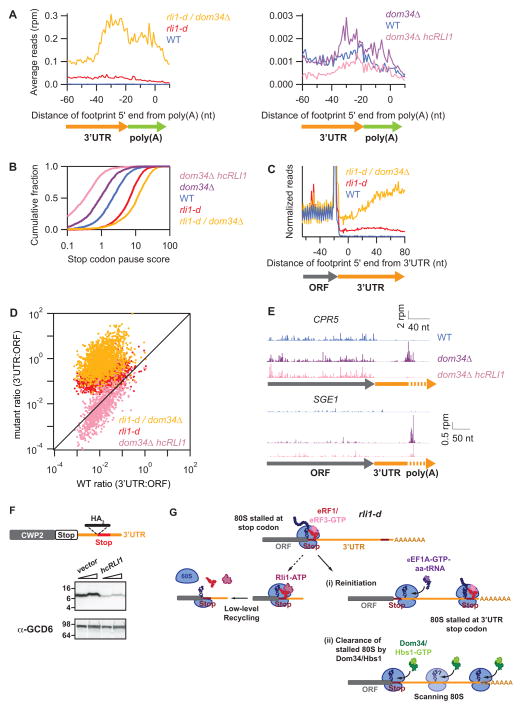
Figure 7. Dom34 is critically required to rescue unrecycled ribosomes in vivo.
(A) Average ribosome occupancy from 3′UTRs aligned at the annotated site of polyadenylation for WT, rli1-d, and rli1-d dom34Δ cells (left panel) and WT, dom34Δ, and dom34Δ/hcRLI1 cells (right panel). (B) Cumulative histogram of pause scores on ORF stop codons computed by taking the ratio of local density at stop codons compared to the overall ORF, for genes with >100 rpkm in ORFs. (C) Normalized average ribosome footprint occupancy from all genes aligned at their stop codons for WT, dom34Δ, rli1-d, and rli1-d dom34Δ strains, analysis as in Fig. 1A. (D) Ratio of footprint densities between 3′UTRs and respective ORFs plotted for the indicated strains, for genes with >5 rpkm in ORFs and >0.5 rpkm in 3′UTRs, with each point representing 1 gene. (E) Ribosome footprints on CPR5 and SGE1. Approximate start site of 3′UTR is indicated. (F) The WT CWP2-3′UTR-HA3 strain was transformed with either empty vector (YEplac195) or hcRLI1 (YEplac195-RLI1). Transformed strains were grown as in Fig. S1A except SCGAL-U and SC-U media was used instead of YPGAL and YPD media to maintain selection for plasmids. WCEs were subjected to Western analysis using antibodies against HA (upper blots) or Gcd6 (lower blots). Two amounts of extracts were loaded in each lane pair.. (G) Schematic model depicting the fate of post-TCs on depletion of Rli1 in rli1-d cells. Recognition of the main ORF stop codon by eRF1/eRF3-GTP (top row) is followed by release of the completed polypeptide and dissociation of eRF3-GDP (not depicted). Any residual Rli1 could bind post-TCs and catalyze dissociation of the 60S subunit (middle row, left). However, many post-TCs are not recycled, migrate a short distance from the stop codon, reinitiate translation, and frequently terminate at a 3′UTR stop codon to produce a 3′UTR-encoded polypeptide (middle row, right). Such reinitiation events appear to be diminished by Dom34, potentially because post-TC ribosomes are rescued at the main ORF stop codons or as they begin scanning. Any ribosomes that reach the 3′UTR/poly(A) boundary by reinitiation or scanning are also rescued by Dom34.