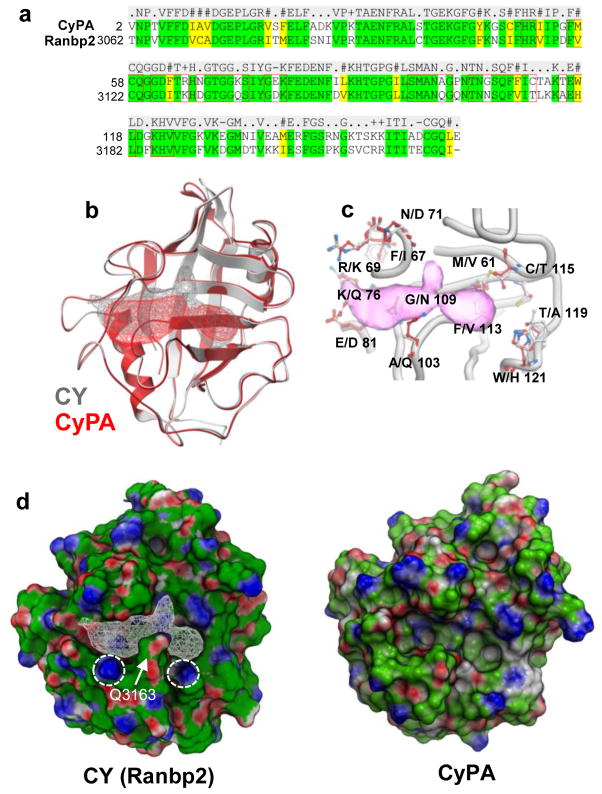
FIGURE 1. Heterogeneity of structural ensembles between the PPIase pockets of CY of Ranbp2 and CyPA.
(a) Amino acid sequence alignment of CY of Ranbp2 and cyclophilin A (CyPA). CY and CyPA are 65% identical. Residues within 7Å of the PPIase pocket are noted within red line boxes. (b) Ribbon representation of superposition of CY of Ranbp2 (gray) and CyPA (red). Mesh represents extended PPIase pocket of CY (gray) and that of CyPA (red). (c) Non-conserved residues of the PPIase/CsA-binding pockets of CyPA (listed first) and CY of Ranbp2 (listed second). Extended CY PPIase pocket is in pink. Numbering refers CyPA residues. (d) Surface representations of CY of Ranbp2 and CyPA colored by qualitative electrostatic potential calculated by ICM using a color scale from red to blue and values of +/−5 kcal/electron units (+5=blue, −5=red). The conserved K3142 and K3185 (circles) and the nonconserved Q3163 residues of CY (arrow) and its PPIase pocket (white mesh) are shown. Variable orientations of side chains of K3142 and K3185 of CY of Ranbp2 and equivalent residues in CyPA cause surface electrostatic shifts.