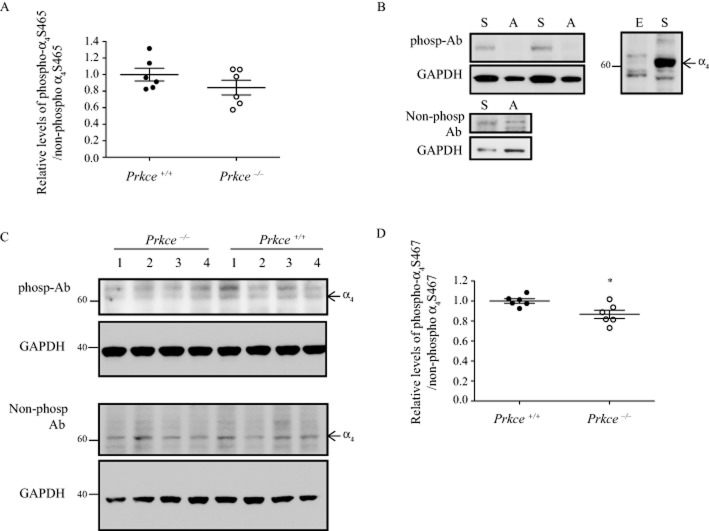
Figure 4.
Phosphorylation of the α4 nAChR subunit in synaptosomes. Phospho-antibodies were raised to the PKCε phosphorylation sites S465 and S467 in the human α4 subunit, corresponding to S468 and S470 in mouse. (A) The level of phosphorylated S465 to non-phosphorylated S465 was not significantly different between wild-type and Prkce−/− mouse synaptosomes. (B) The S467 phospho-antibody detected a strong band from HEKtsA201 cells expressing native α4β2 nAChRs ‘S’ and did not detect a band from cells expressing the S467A mutation ‘A’. The non-phospho-α4 antibody detected bands from both native and S467A mutant receptors. Corresponding GAPDH levels are shown in the lower panel. The S467 phospho-antibody detected several non-specific bands in an overexposure from HEKtsA201 cells that do not express α4β2 nAChRs ‘E’. (C) The phospho-α4 S467 and non-phospho-α4 S467 antibodies detected bands in synaptosome preparations from wild-type and Prkce−/− mouse brains. The corresponding GAPDH blots are shown below each panel. The 60 kDa molecular weight band was quantified from the phospho-α4 S467 blots. Molecular weights are indicated on the side (kDa). (D) The level of phosphorylated α4 S467 to non-phosphorylated α4 S467 was 13% higher in synaptosomes from wild-type compared with Prkce−/− mouse brains. *P < 0.05 compared with wild-type mice, n = 6 mice per genotype.