Abstract
Researchers have examined representations of the body in the adult brain, but relatively little attention has been paid to ontogenetic aspects of neural body maps in human infants. Novel applications of methods for recording brain activity in infants are delineating cortical body maps in the first months of life. Body maps may facilitate infants’ registration of similarities between self and other—an ability that is foundational to developing social cognition. Alterations in interpersonal aspects of body representations might also contribute to social deficits in certain neurodevelopmental disorders.
Keywords: Body maps, infancy, development, body schema, social neuroscience, somatotopy
Connecting Self and Other through Neural Body Representations
The past decade has seen sustained interest in the neural processes involved in the perception of the human body. Studies in adults have illuminated the brain networks contributing to the sense of body ownership [1, 2] and have documented cortical regions associated with perceiving bodies of other people [3–5].
Historically, the representation of one’s own body and the perception of the bodies of others have often been studied independently. Growing attention is now being paid to the interconnections between them [6–8], including the role of neural representations of the body in adult social cognition. There is evidence that the brain systems mediating the perceptual and sensory experience of one’s own body are involved in social and emotional processes [9–14].
Although research with adults is providing insights into interpersonal aspects of body representations, developmental studies are lacking. One line of relevant behavioral research has examined infants’ visual recognition of human forms; but much of this work has not considered infants’ representation of their own body or how this might influence the perception and representation of the bodies of others (Box 1). Here we focus on somatotopic maps (see Glossary) in the infant brain as a foundational aspect of how the body is represented in early human development. Novel applications of methods for recording infant brain activity can foster an understanding of how cortical body maps emerge and develop and can illuminate their role in facilitating connections between self and other in the first weeks and months of life.
Box 1. Human Infants’ Responses to Depictions of the Human Body.
There is increasing interest in studying developmental aspects of body representations [80]. Initial studies suggested that sensitivity to body structure in static images was not apparent until the second year of life [81]. However, recent behavioral work suggests that infants may be sensitive to disruptions in the configuration of the human body at significantly younger ages [82–84]. Accompanying these behavioral investigations is a small number of studies examining infant neural responses to experimentally manipulated disruptions in bodily representations in static and dynamic displays [85–88]. In terms of how infants represent their own bodies in relation to the bodies of others, recent behavioral work shows that newborns detect temporal and spatial correspondences between a video display of an infant’s face being stroked and tactile stimulation of their own face [89, 90]. An investigation using fNIRS suggests that temporal lobe activity differs in 5-month-old infants who viewed video displays of their body that were temporally contingent with their movements versus delayed [17].
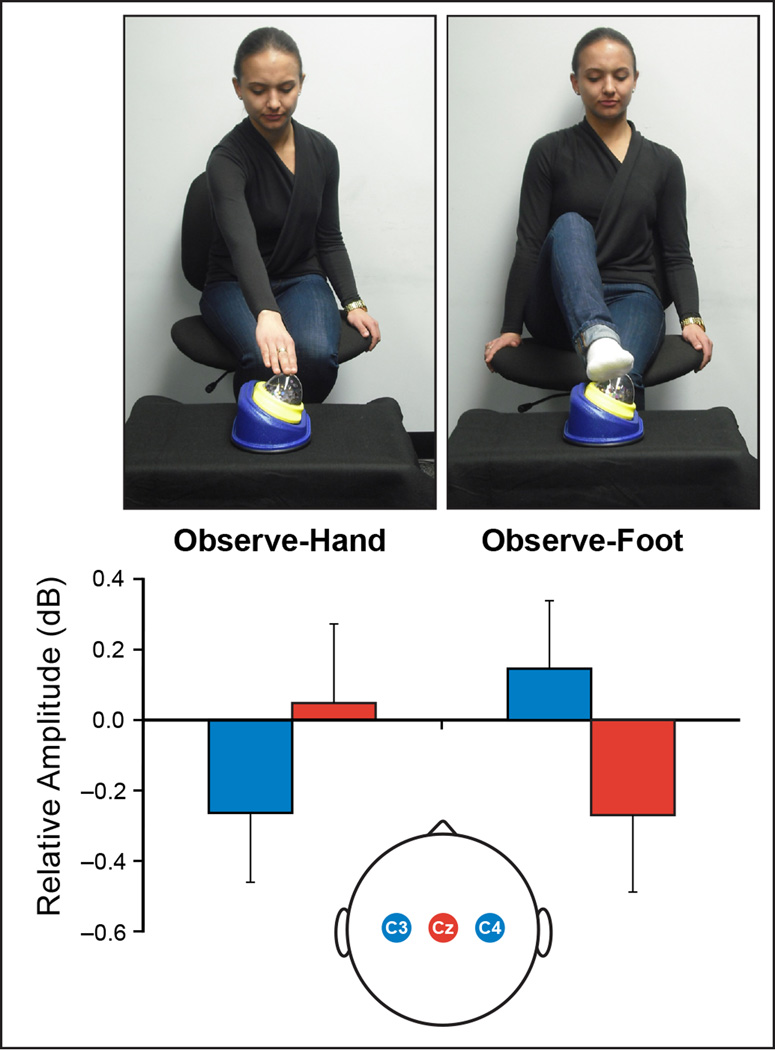
Alongside research programs using infant magnetoencephalography (MEG) [15, 16] and functional near-infrared spectroscopy (fNIRS) [17, 18], refinements in electroencephalography (EEG) are stimulating new investigations of the neural processes involved in early social engagement [19–24]. One set of EEG findings highlighting social implications of body maps comes from studies showing that the sensorimotor mu rhythm displays a somatotopic response pattern during both action observation and action production in 14-month-old infants [25, 26] (Figure 1). This provides neuroscience evidence that infants can register correspondences between their own body parts and the body parts of others. It also invites further studies of somatotopic organization in the infant brain, and how it relates to key aspects of human development, including imitation [24, 27].
Figure 1. Somatotopic mu rhythm responses to action observation in 14-month-olds.
Infants watched an adult reach towards and touch an object using either her hand or her foot. The goal of touching the domed surface was the same but the body part used was different. The pattern of activation over hand (electrodes C3/C4) and foot (electrode Cz) regions of sensorimotor cortex differed significantly according to whether infants saw a hand or a foot used. For hand actions there was a greater reduction in mu amplitude over C3/C4 (blue bars); conversely, for foot actions there was greater reduction in mu amplitude at Cz (red bars). Figure is adapted from [26].
Research on the mu rhythm continues to shed light on how infants perceive the actions of others in relation to their own capacities for action [24, 28, 29]. Here we outline a new direction for infant cognitive neuroscience that brings attention to aspects of neural organization that have been missed or underemphasized by the focus on the motor cortex and “mirror neurons” in past developmental work (see Box 2). We describe ideas and research that are employing methods for recording cortical responses to somatosensory stimulation in order to probe the development of body maps in the brain. These methods for investigating body maps in human infancy provide connections to fertile areas of neuroscience research in adults on somatotopy, plasticity, and cortical representations of the body. Although much is known about neural somatotopy in adults, developmental work in this area is virtually nonexistent. The novel idea being advanced here is that as one aspect of the developing body schema, body maps in the infant brain are involved in the basic registration of self-other correspondences, and as such they may facilitate the earliest relationships and feelings of connectedness with others. We articulate a developmental position that addresses questions of neural plasticity and provides a fresh view on crucial aspects of social-cognitive development (see Open Questions).
Box 2. Multiple Body Maps in the Brain.
Neural maps have been found in multiple areas of the mammalian brain, including motor and somatosensory cortices [91]. Classical work on these maps in humans and other species focused on responses to tactile simulation and execution of motor actions. Researchers have increasingly investigated the activation of motor areas during action observation, including somatotopically organized neural responses in adults during the viewing of the bodily movements of other people [92]. Alongside this line of research, a newer literature is emerging that highlights the role of somatosensory cortex in social perception in adults, including somatotopic representations [9]. Most neuroscience studies of human infants have not caught up with the idea that the bodily representation in somatosensory cortex may play a role in processing social signals about others. For instance, much of the extant research on the infant EEG mu rhythm has been interpreted within a motor framework. However, the mu rhythm is not solely (or even primarily) a motor rhythm [78, 93, 94]. The emphasis on motor influences may be unnecessarily limiting for work with human infants; and broadening the view to include somatosensory processes in social perception promises to advance developmental theory [24, 27]. Neuroscience investigations of infants will profit from considering multiple body maps (both somatosensory and motor) and their interactions (see Outstanding Questions).
Neuroscience Approaches to Investigating Body Maps
Research on the representation of the body in the mammalian brain has often focused on the properties of somatotopic maps in primary somatosensory cortex (SI). Extensive research with human adults as well as with nonhuman primates has examined questions concerning change in body maps in SI in response to changes in afferent input [30, 31] and learning [32, 33]. However, despite a large literature on somatotopic body maps in adults, little attention has been paid to how these maps are established and refined. Some developmental insights may be gleaned from neuroscience research with nonhuman species (Box 3) as well as from computational approaches. Although these existing lines of research can provide guideposts, advancing our understanding of the ontogenesis of body maps in the human brain requires the application of non-invasive brain imaging methods with young infants.
Box 3. Insights from Animal Models.
Classic behavioral neuroscience work on whisker barrels highlighted the role of afferent input in the somatotopic organization of rodent somatosensory cortex [95], with more recent research illuminating the specific mechanisms involved [96, 97]. Other work with rodents suggests that the development of somatotopy depends on sensory feedback from muscle twitches that are spontaneously generated by the spinal cord and subcortical structures [98, 99]. A similar mechanism has been suggested to play a role in sensorimotor development in humans [42].
Top-down influences have also been shown to be important [100]. In both juvenile and adult monkeys, severing the median nerve (which carries sensory information from the hand to the brain) results in a haphazard pattern of innervation of the skin surface. This disordered pattern is propagated through afferent pathways to SI, where a jumbled representation of the hand replaces the normal somatotopic arrangement [101]. However, if the median nerve is severed very early in development, a considerable degree of somatotopy is retained in SI [102], suggesting that early in development, an existing cortical body map can direct the reorganization of thalamocortical projections following a disruption in the orderly patterning at the periphery [103].
One promising approach for delineating cortical body maps in infants involves examining the topography of event-related responses to tactile stimulation applied to different parts of the body. The analysis of EEG and MEG responses to somatosensory stimulation has been useful for investigating neural body maps in adults [34–37] and is proving valuable for ontogenetic work. A number of studies with infants have examined responses that are evoked by stimulation of one or both hands [38–41]. These responses are typically strongest at central electrode sites in the contralateral hemisphere, which echoes findings in adults and is suggestive of a somatotopic organization of responses to tactile stimulation.
Although studies involving hand stimulation have been informative, a fuller delineation of infant body maps depends on the collection of brain responses to stimulation of a wider range of body parts. In two EEG studies of preterm newborns, tactile stimulation of the hands was associated with visibly increased oscillatory activity at lateral central electrodes, while stimulation of the feet was associated with increased activity at the midline central electrode [42, 43]. The stimuli used in these studies were relatively uncontrolled in terms of their precise location, intensity, and duration, and the unusual profile of the preterm EEG signal [44] precludes comparisons with the brain responses of older children and adults.
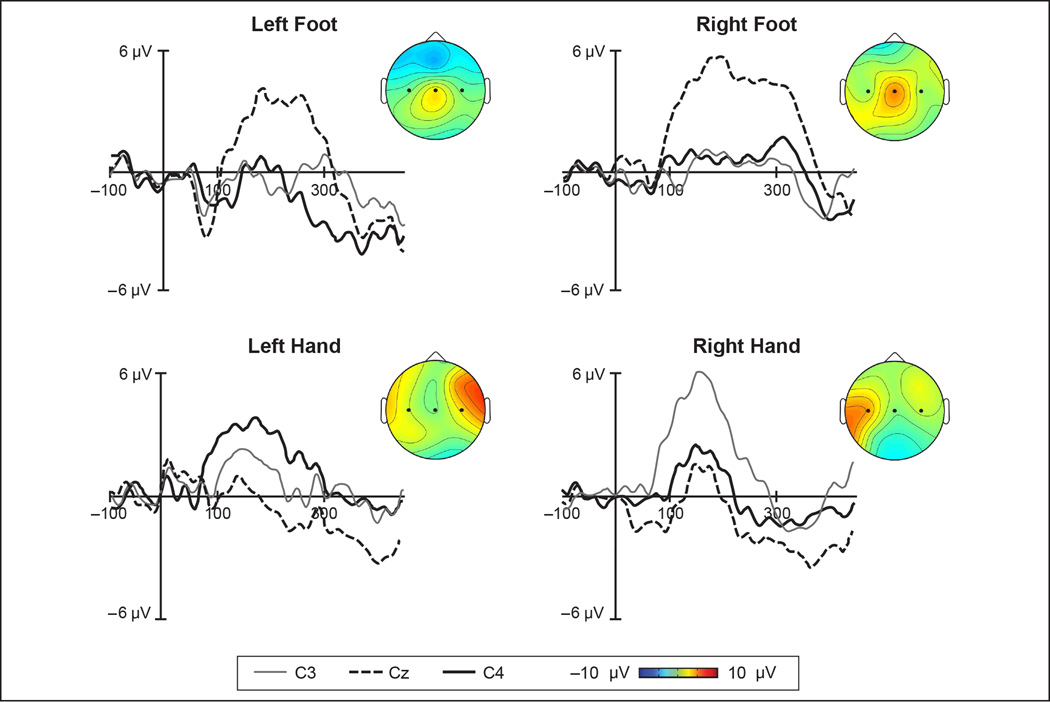
In recent work we recorded the somatosensory evoked potential (SEP) elicited in response to brief (60 ms) tactile stimuli that were applied to specific areas on the hands and feet of 7-month-old infants using precisely controlled delivery methods [45]. Analyses focused on the magnitude of a prominent positivity in the SEP that peaked around 175 ms following onset of the tactile stimulus (Figure 2). In response to hand stimulation, the amplitude of this positive component was significantly larger over the lateral central electrodes (C3 and C4) than over the midline central electrode (Cz). In contrast, stimulation of the foot elicited a significantly larger response at the midline site than at the lateral sites. This indication of somatotopy in the infant SEP response suggests that measuring event-related scalp responses to brief, discrete tactile stimulation using EEG provides a practical and informative method for mapping the representation of the body in the infant brain. The ability to successfully elicit SEP responses from multiple body parts in awake infants also opens up novel possibilities for examining neural aspects of cross-modal interactions (e.g., visuo-tactile) in early development.
Figure 2. Somatosensory evoked potentials elicited to tactile stimulation of 7-month-old infants’ hands and feet.
Discrete, computer-controlled tactile stimulation elicited a large positive component peaking around 175 ms that was organized somatotopically over central electrode sites. For left and right hand stimulation, amplitudes were greatest at lateral electrodes (C3/C4). For left and right foot stimulation, peak amplitude was greatest at the midline central electrode (Cz). Topographic maps show amplitude distribution across the scalp. Figure is adapted and reprinted from [45] with permission from Elsevier.
Developing Body Maps in the Human Brain
We suggest that continued research on neural somatotopic organization in infancy can illuminate the mechanisms of social engagement that are crucial for typical human development [46]. In particular, research on infant body maps promises to shed light on a fundamental question in developmental psychology: How human infants develop a sense of themselves as individuals who are both similar to, and at the same time distinct from, other people. One psychological model of young children’s social development, the “Like-Me” framework [47, 48], proposes that infants gain an initial foothold on the social world through a felt similarity between their own bodily acts and the bodily acts of others. The perception of this similarity depends on a primitive body schema that is influenced by self-generated bodily activity beginning prenatally [27]. Infants are hypothesized to use this experience to generate an act space that links the behavior of others to the behavior of self through shared representations [49]. Although this claim has been made at a theoretical level, and there are supportive behavioral data from the study of imitation, there has been little exploration of the neural processes involved in the social matching behavior that constitutes infant imitation.
One way of advancing the understanding of connections between neural body maps and social processes is to examine whether cortical responses to tactile stimulation can be modulated by selective attention to the self or other. For adults, devoting attention to one’s own hand modifies cortical responses to tactile stimulation [50], with effects at early stages of processing that likely reflect top-down modulation of primary somatosensory cortex [51]. Moreover, adult EEG responses to tactile stimulation are modulated according to the congruence between the stimulated body part and the observation of a matching or different body part of another person [52, 53]. An MEG study with older children investigated responses to tactile stimulation of the index finger during the presentation of video clips showing another person being touched on either the hands or feet [54]. Activation over the hand areas of somatosensory cortex was strongest when the tactile stimulation occurred during viewing of the hand stimulation videos. This suggests possibilities for examining whether a similar modulation is present in infants.
It readily becomes apparent that there are a host of other novel questions about developing body maps in human infants (see Outstanding Questions). One interesting issue concerns the spatial resolution at which body parts can be mapped in the infant brain. The extant developmental EEG research has used low-density scalp arrays and has involved body parts that are relatively distant from each other in cortical body maps. High-density EEG and MEG studies involving the stimulation of multiple body parts will prove useful for building a more detailed developmental picture of somatotopic neural organization.
Outstanding Questions.
Can non-invasive neuroscience methods with high spatial resolution (e.g., MEG) be adapted to study the ontogenesis of fine-grained somatotopic organization of body maps in human infants? How many body parts can be clearly differentiated in infant neural body maps?
Can advanced tools such as MEG differentiate between somatosensory and motor maps in human infants and illuminate the interactions between these maps?
What are the neural temporal dynamics that occur when infants experience tactile stimulation?
Do body maps show neuroplasticity in relation to changes in body growth and behavioral abilities, including developmental progressions in reaching, grasping, goal-directed acts, and expertise in tool use?
How can neuroscience research on infant body maps be more deeply interwoven with psychological theorizing about the early development of the body schema and its role in social-emotional and cognitive development?
Another question concerns how the organization of body maps may shift with changes in body morphology and development. Given the transformations in behavioral skills that occur in infancy—including in grasping, crawling, and walking—infancy is an ideal period in which to explore questions about the effects of experience on neural body maps. There is some evidence that changes in the neural response to hand stimulation are correlated with developments in infants’ reaching and grasping abilities [41, 55]. A related topic concerns the effects of learning to use tools, a key part of infant development [56]. Expert tool use alters aspects of the body schema in both nonhuman primates [57] and human adults [58], and may do so in the developing infant brain. Another interesting question concerns how somatotopic representations combine with information about moment-to-moment changes in the positions of body parts as well as with representations of external (e.g., peripersonal) space [45].
Studying the ontogenetic aspects of body maps may also have implications for the study of atypical development, particularly autism spectrum disorder (ASD). It is clear that ASD is a heterogeneous, complex syndrome and that single-cause explanations will not suffice. At a broad level, however, it has been suggested that ASD involves a disruption in self-other processing that affects the ability to form and co-ordinate social representations, with cascading effects on imitation, communication, and interpersonal interaction [59]. Investigations of the role of neural body maps in facilitating self-other correspondences can complement ongoing lines of research on body representations in children with ASD [60–64]. Future exploratory work on the role of neural body maps in establishing interpersonal linkages has the potential to illuminate the mechanisms involved in interventions for ASD that emphasize bodily-action coordination and mutual imitation between children and therapists [65–68].
Concluding Remarks
Researchers across the rapidly growing field of developmental cognitive neuroscience are addressing a variety of questions at the interface of brain, behavior, cognition, and developmental processes [69–71]. The inherent complexity of this interface necessitates integrative approaches in which findings from different domains can be leveraged to make informed predictions about brain-behavior relations in human ontogenesis [72]. We suggest that developmentally-oriented research on neural body maps holds particular promise for building bridges between literatures on the neural somatotopic organization in nonhuman species, adult cognitive neuroscience, and an emerging literature on body representations in human infancy. As such, the study of infant body maps provides an ideal domain for examining developmental brain-behavior relations and neuroplasticity in humans.
We have highlighted a possible role of neural body maps in facilitating the registration of correspondences between self and other in early human development, prior to language. Further work in this area can advance the understanding of essential aspects of infant cognition such as the ability to imitate and learn from others via observation [73]. One important component of imitation involves visually identifying the part of the body used by another person to carry out an action, and then selecting the corresponding body part on one’s own body to generate the imitative response [27, 74]. The neural systems involved in the development of imitation remain under study. It is possible that body maps play a fundamental role in the processes that allow this powerful mechanism of social learning to unfold in human infancy.
Highlights.
Body representations in the human brain are studied chiefly in adults.
Recent discoveries are shedding light on body maps in the infant brain.
Infant neural body maps underlie early connections between self and other.
Studies of infant neural body maps may illuminate certain developmental disorders.
Acknowledgements
Support for the writing of this article came from NSF awards BCS-1460889 and SMA-1540619, NIH award R21HD083756, and the Institute for Learning & Brain Sciences Ready Mind Project Fund. We thank Joni Saby for helpful discussions.
Glossary
- Body schema
In humans, the body maps of preverbal children can be viewed as one building block of the complex psychological construct of the body schema, which refers to sensorimotor representations of the body that guide actions without awareness or the necessity of conscious monitoring [75]. The body schema is distinct from the concept of the body image, which is a later-occurring psychological achievement and refers to more conceptual, consciously accessible aspects of bodily awareness, including culturally appropriate appearances [76, 77].
- Mu rhythm
A brain oscillation in the alpha frequency range (8–13 Hz in adults, slightly lower frequencies in infants and children, e.g., 6–9 Hz) that can be detected over sensorimotor regions using EEG and MEG methods. The mu rhythm is desynchronized (reduced in amplitude) during action observation and action production in infants, children, and adults [24, 28, 78].
- Somatosensory Evoked Potential (SEP)
An averaged, time-locked response in the EEG signal at central electrode sites that is elicited by somatosensory stimulation. The SEP response can be elicited by various means including median nerve stimulation and tactile stimulation of the skin. The analogous response in the MEG signal is the somatosensory evoked field (SEF).
- Somatotopic map
A spatial arrangement of neurons reflecting the topography of body parts. One well-studied example of a somatotopic map in the brain of humans and nonhuman primates is the homuncular representation of the body surface in primary somatosensory cortex (SI) [79]. The representation of the body surface in SI represents the endpoint of sensory pathways carrying information about touch (from the skin) and proprioception (from the joints of the body). These projections retain an orderly somatotopic organization as they ascend to SI from the periphery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Peter J. Marshall, Department of Psychology, Temple University, Philadelphia, PA, USA.
Andrew N. Meltzoff, Institute for Learning & Brain Sciences, University of Washington, Seattle, WA, USA
References Cited
- 1.Tsakiris M, et al. Having a body versus moving your body: Neural signatures of agency and body-ownership. Neuropsychologia. 2010;48:2740–2749. doi: 10.1016/j.neuropsychologia.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Brugger P, Lenggenhager B. The bodily self and its disorders: Neurological, psychological and social aspects. Curr. Opin. Neurol. 2014;27:644–652. doi: 10.1097/WCO.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 3.Schwarzlose RF, et al. Separate face and body selectivity on the fusiform gyrus. J. Neurosci. 2005;25:11055–11059. doi: 10.1523/JNEUROSCI.2621-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downing PE, Peelen MV. The role of occipitotemporal body-selective regions in person perception. Cogn. Neurosci. 2011;2:186–203. doi: 10.1080/17588928.2011.582945. [DOI] [PubMed] [Google Scholar]
- 5.de Gelder B, et al. Standing up for the body. Recent progress in uncovering the networks involved in the perception of bodies and bodily expressions. Neurosci. Biobehav. Rev. 2010;34:513–527. doi: 10.1016/j.neubiorev.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 6.de Vignemont F. Shared body representations and the 'Whose' system. Neuropsychologia. 2014;55:128–136. doi: 10.1016/j.neuropsychologia.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Reed CL. Seeing you through me: Creating self-other correspondences for body perception. In: Johnson KL, Shiffrar M, editors. People watching: Social, perceptual, and neurophysiological studies of body perception. Oxford University Press; 2013. pp. 44–62. [Google Scholar]
- 8.Berlucchi G, Aglioti SM. The body in the brain revisited. Exp. Brain Res. 2010;200:25–35. doi: 10.1007/s00221-009-1970-7. [DOI] [PubMed] [Google Scholar]
- 9.Keysers C, et al. Somatosensation in social perception. Nat. Rev. Neurosci. 2010;11:417–428. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- 10.Kuehn E, et al. The functional architecture of S1 during touch observation described with 7 T fMRI. Brain Struct. Funct. 2014;219:119–140. doi: 10.1007/s00429-012-0489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sel A, et al. The emotional homunculus: ERP evidence for independent somatosensory responses during facial emotional processing. J. Neurosci. 2014;34:3263–3267. doi: 10.1523/JNEUROSCI.0106-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolognini N, et al. Sharing social touch in the primary somatosensory cortex. Curr. Biol. 2014;24:1513–1517. doi: 10.1016/j.cub.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Damasio A, Carvalho GB. The nature of feelings: Evolutionary and neurobiological origins. Nat. Rev. Neurosci. 2013;14:143–152. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- 14.Jackson PL, et al. How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Bosseler AN, et al. Theta brain rhythms index perceptual narrowing in infant speech perception. Front. Psych. 2013;4:690. doi: 10.3389/fpsyg.2013.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhl PK, et al. Infants’ brain responses to speech suggest analysis by synthesis. Proc. Natl. Acad. Sci. U. S. A. 2014;111:11238–11245. doi: 10.1073/pnas.1410963111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filippetti ML, et al. Neural mechanisms of body awareness in infants. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu261. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aslin RN, et al. Hemodynamic correlates of cognition in human infants. Annu. Rev. Psychol. 2015;66:349–379. doi: 10.1146/annurev-psych-010213-115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saby JN, Marshall PJ. The utility of EEG band power analysis in the study of infancy and early childhood. Dev. Neuropsychol. 2012;37:253–273. doi: 10.1080/87565641.2011.614663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Righi G, et al. Functional connectivity in the first year of life in infants at risk for autism spectrum disorder: An EEG study. PLOS ONE. 2014;9:pe105176. doi: 10.1371/journal.pone.0105176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bathelt J, et al. Functional brain network organisation of children between 2 and 5 years derived from reconstructed activity of cortical sources of high-density EEG recordings. NeuroImage. 2013;82:595–604. doi: 10.1016/j.neuroimage.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Bell MA, Cuevas K. Using EEG to study cognitive development: Issues and practices. J. Cogn. Dev. 2012;13:281–294. doi: 10.1080/15248372.2012.691143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Missana M, et al. Tuning the developing brain to emotional body expressions. Dev. Sci. 2015;18:243–253. doi: 10.1111/desc.12209. [DOI] [PubMed] [Google Scholar]
- 24.Marshall PJ, Meltzoff AN. Neural mirroring mechanisms and imitation in human infants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130620. doi: 10.1098/rstb.2013.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall PJ, et al. Imitation and the developing social brain: Infants’ somatotopic EEG patterns for acts of self and other. Int. J. Psychol. Res. 2013;6:22–29. doi: 10.21500/20112084.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saby JN, et al. Infants’ somatotopic neural responses to seeing human actions: I’ve got you under my skin. PLOS ONE. 2013;8:e77905. doi: 10.1371/journal.pone.0077905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meltzoff AN, Moore MK. Explaining facial imitation: A theoretical model. Early Dev. Parent. 1997;6:179–192. doi: 10.1002/(SICI)1099-0917(199709/12)6:3/4<179::AID-EDP157>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuevas K, et al. The infant EEG mu rhythm: Methodological considerations and best practices. Dev. Rev. 2014;34:26–43. doi: 10.1016/j.dr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall PJ, Meltzoff AN. Neural mirroring systems: Exploring the EEG mu rhythm in infancy. Dev. Cogn. Neurosci. 2011;1:110–123. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaas JH. Plasticity of sensory and motor maps in adult mammals. Annu. Rev. Neurosci. 1991;14:137–167. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran VS, Rogers-Ramachandran D. Phantom limbs and neural plasticity. Arch. Neurol. 2000;57:317–320. doi: 10.1001/archneur.57.3.317. [DOI] [PubMed] [Google Scholar]
- 32.Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu. Rev. Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 33.Elbert T, et al. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 34.Heed T, Roder B. Common anatomical and external coding for hands and feet in tactile attention: Evidence from event-related potentials. J. Cogn. Neurosci. 2010;22:184–202. doi: 10.1162/jocn.2008.21168. [DOI] [PubMed] [Google Scholar]
- 35.Dowman R, Schell S. Innocuous-related sural nerve-evoked and finger-evoked potentials generated in the primary somatosensory and supplementary motor cortices. Clin. Neurophysiol. 1999;110:2104–2116. doi: 10.1016/s1388-2457(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura A, et al. Somatosensory homunculus as drawn by MEG. NeuroImage. 1998;7:377–386. doi: 10.1006/nimg.1998.0332. [DOI] [PubMed] [Google Scholar]
- 37.Hari R, et al. Functional organization of the human first and second somatosensory cortices: a neuromagnetic study. Eur. J. Neurosci. 1993;5:724–734. doi: 10.1111/j.1460-9568.1993.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 38.Hrbek A, et al. Development of visual and somatosensory evoked responses in pre-term newborn infants. Electroencephalogr. Clin. Neurophysiol. 1973;34:225–232. doi: 10.1016/0013-4694(73)90249-6. [DOI] [PubMed] [Google Scholar]
- 39.Pihko E, et al. Maturation of somatosensory cortical processing from birth to adulthood revealed by magnetoencephalography. Clin. Neurophysiol. 2009;120:1552–1561. doi: 10.1016/j.clinph.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 40.Nevalainen P, et al. Evaluation of somatosensory cortical processing in extremely preterm infants at term with MEG and EEG. Clin. Neurophysiol. 2015;126:275–283. doi: 10.1016/j.clinph.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 41.Rigato S, et al. The neural basis of somatosensory remapping develops in human infancy. Curr. Biol. 2014;24:1222–1226. doi: 10.1016/j.cub.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Milh M, et al. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb. Cortex. 2007;17:1582–1594. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- 43.Vanhatalo S, et al. An easy and practical method for routine, bedside testing of somatosensory systems in extremely low birth weight infants. Pediatr. Res. 2009;66:710–713. doi: 10.1203/PDR.0b013e3181be9d66. [DOI] [PubMed] [Google Scholar]
- 44.Nevalainen P, et al. Development of human somatosensory cortical functions. What have we learned from magnetoencephalography? Front. Hum. Neurosci. 2014;8:158. doi: 10.3389/fnhum.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saby JN, et al. Neural body maps in human infants: Somatotopic responses to tactile stimulation in 7-month-olds. NeuroImage. 2015;118:74–78. doi: 10.1016/j.neuroimage.2015.05.097. [DOI] [PubMed] [Google Scholar]
- 46.Rochat P. Others in mind: Social origins of self-consciousness. Cambridge University Press; 2009. [Google Scholar]
- 47.Meltzoff AN. ‘Like me’: A foundation for social cognition. Dev. Sci. 2007;10:126–134. doi: 10.1111/j.1467-7687.2007.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meltzoff AN. Origins of social cognition: Bidirectional self-other mapping and the “Like-Me” hypothesis. In: Banaji M, Gelman S, editors. Navigating the social world: What infants, children, and other species can teach us. Oxford University Press; 2013. pp. 139–144. [Google Scholar]
- 49.Meltzoff AN. The ‘like me’ framework for recognizing and becoming an intentional agent. Acta Psychol. 2007;124:26–43. doi: 10.1016/j.actpsy.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambo CF, et al. Viewing the body modulates neural mechanisms underlying sustained spatial attention in touch. Eur. J. Neurosci. 2009;30:143–150. doi: 10.1111/j.1460-9568.2009.06791.x. [DOI] [PubMed] [Google Scholar]
- 51.Longo MR, et al. Vision of the body modulates processing in primary somatosensory cortex. Neurosci. Lett. 2011;489:159–163. doi: 10.1016/j.neulet.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Voisin JI, et al. I am touched by your pain: Limb-specific modulation of the cortical response to a tactile stimulation during pain observation. J. Pain. 2011;12:1182–1189. doi: 10.1016/j.jpain.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Schaefer M, et al. Seeing the hand being touched modulates the primary somatosensory cortex. Neuroreport. 2005;16:1101–1105. doi: 10.1097/00001756-200507130-00014. [DOI] [PubMed] [Google Scholar]
- 54.Remijn GB, et al. Somatosensory evoked field in response to visuotactile stimulation in 3- to 4-year-old children. Front. Hum. Neurosci. 2014;8:170. doi: 10.3389/fnhum.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gondo K, et al. A magnetoencephalographic study on development of the somatosensory cortex in infants. Neuroreport. 2001;12:3227–3231. doi: 10.1097/00001756-200110290-00017. [DOI] [PubMed] [Google Scholar]
- 56.Kahrs BA, Lockman JJ. Tool using. Child Dev. Perspect. 2014;8:231–236. doi: 10.1111/cdep.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maravita A, Iriki A. Tools for the body (schema) Trends Cogn. Sci. 2004;8:79–86. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Miller LE, et al. Tool morphology constrains the effects of tool use on body representations. J. Exp. Psychol. Hum. Percept. Perform. 2014;40:2143–2153. doi: 10.1037/a0037777. [DOI] [PubMed] [Google Scholar]
- 59.Rogers SJ, Williams JHG. Imitation and the social mind: Autism and typical development. Guilford Press; 2006. [Google Scholar]
- 60.Malinen S, et al. Functional parcellation of the human primary somatosensory cortex to natural touch. Eur. J. Neurosci. 2014;39:738–743. doi: 10.1111/ejn.12493. [DOI] [PubMed] [Google Scholar]
- 61.Gallace A, Spence C. The science of interpersonal touch: An overview. Neurosci. Biobehav. Rev. 2010;34:246–259. doi: 10.1016/j.neubiorev.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Cascio CJ, et al. The rubber hand illusion in children with autism spectrum disorders: Delayed influence of combined tactile and visual input on proprioception. Autism. 2012;16:406–419. doi: 10.1177/1362361311430404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schauder KB, et al. Interoceptive ability and body awareness in autism spectrum disorder. J. Exp. Child Psychol. 2015;131:193–200. doi: 10.1016/j.jecp.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaiser MD, et al. Brain mechanisms for processing affective (and nonaffective) touch are atypical in autism. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vivanti G, Rogers SJ. Autism and the mirror neuron system: Insights from learning and teaching. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014;369:20130184. doi: 10.1098/rstb.2013.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ingersoll B. Pilot randomized controlled trial of Reciprocal Imitation Training for teaching elicited and spontaneous imitation to children with autism. J. Autism Dev. Disord. 2010;40:1154–1160. doi: 10.1007/s10803-010-0966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berger NI, Ingersoll B. An evaluation of imitation recognition abilities in typically developing children and young children with Autism Spectrum Disorder. Autism Res. 2015 doi: 10.1002/aur.1462. [DOI] [PubMed] [Google Scholar]
- 68.Nadel J. How imitation boosts development in infancy and autism spectrum disorder. Oxford University Press; 2014. [Google Scholar]
- 69.Johnson MH, De Haan M. Developmental cognitive neuroscience. 4th ed. Wiley-Blackwell; 2015. [Google Scholar]
- 70.Nelson CA, Luciana M. Handbook of developmental cognitive neuroscience. 2nd ed. MIT Press; 2008. [Google Scholar]
- 71.Blakemore SJ, et al. Developmental cognitive neuroscience. Dev. Cogn. Neurosci. 2011;1:3–6. doi: 10.1016/j.dcn.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marshall PJ. Neuroscience, embodiment, and development. In: Overton WF, et al., editors. Vol. 1 of Handbook of child psychology and developmental science. Wiley; 2015. pp. 244–283. [Google Scholar]
- 73.Meltzoff AN, et al. Foundations for a new science of learning. Science. 2009;325:284–288. doi: 10.1126/science.1175626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meltzoff AN. Infant imitation after a 1-week delay: Long-term memory for novel acts and multiple stimuli. Dev. Psychol. 1988;24:470–476. doi: 10.1037/0012-1649.24.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gallagher S. How the body shapes the mind. Oxford University Press; 2005. [Google Scholar]
- 76.Gallagher S, Meltzoff AN. The earliest sense of self and others: Merleau-Ponty and recent developmental studies. Philos. Psychol. 1996;9:211–233. doi: 10.1080/09515089608573181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Vignemont F. Body schema and body image - Pros and cons. Neuropsychologia. 2010;48:669–680. doi: 10.1016/j.neuropsychologia.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 78.Hari R. Action-perception connection and the cortical mu rhythm. Prog. Brain Res. 2006;159:253–260. doi: 10.1016/S0079-6123(06)59017-X. [DOI] [PubMed] [Google Scholar]
- 79.Kaas JH, et al. The organization of the somatosensory system in primates. In: Nelson RJ, editor. The somatosensory system: Deciphering the brain’s own body image. CRC Press; 2002. pp. 1–25. [Google Scholar]
- 80.Slaughter V, Brownell CA. Early development of body representations. Cambridge University Press; 2012. [Google Scholar]
- 81.Slaughter V, Heron M. Origins and early development of human body knowledge. Monogr. Soc. Res. Child Dev. 2004;69 doi: 10.1111/j.0037-976X.2004.00286.x. (2, Serial No. 276) [DOI] [PubMed] [Google Scholar]
- 82.Zieber N, et al. Body structure perception in infancy. Infancy. 2015;20:1–17. [Google Scholar]
- 83.Heron-Delaney M, et al. Infants’ knowledge of their own species. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011;366:1753–1763. doi: 10.1098/rstb.2010.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Longhi E, et al. Discrimination of biomechanically possible and impossible hand movements at birth. Child Dev. 2015;86:632–641. doi: 10.1111/cdev.12329. [DOI] [PubMed] [Google Scholar]
- 85.Marshall PJ, Shipley TF. Event-related potentials to point-light displays of human actions in 5-month-old infants. Dev. Neuropsychol. 2009;34:368–377. doi: 10.1080/87565640902801866. [DOI] [PubMed] [Google Scholar]
- 86.Reid VM, et al. The perception of biological motion by infants: An event-related potential study. Neurosci. Lett. 2006;395:211–214. doi: 10.1016/j.neulet.2005.10.080. [DOI] [PubMed] [Google Scholar]
- 87.Gliga T, Dehaene-Lambertz G. Structural encoding of body and face in human infants and adults. J. Cogn. Neurosci. 2005;17:1328–1340. doi: 10.1162/0898929055002481. [DOI] [PubMed] [Google Scholar]
- 88.Grossmann T, et al. Action observation in the infant brain: The role of body form and motion. Soc. Neurosci. 2012;8:22–30. doi: 10.1080/17470919.2012.696077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Filippetti ML, et al. Body perception in newborns. Curr. Biol. 2013;23:2413–2416. doi: 10.1016/j.cub.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Filippetti ML, et al. Newborn body perception: Sensitivity to spatial congruency. Infancy. 2015 doi: 10.1111/infa.12083. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaas JH. Topographic maps are fundamental to sensory processing. Brain Res. Bull. 1997;44:107–112. doi: 10.1016/s0361-9230(97)00094-4. [DOI] [PubMed] [Google Scholar]
- 92.Buccino G, et al. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur. J. Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- 93.Arnstein D, et al. Mu-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J. Neurosci. 2011;31:14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ritter P, et al. Rolandic alpha and beta EEG rhythms' strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum. Brain Mapp. 2009;30:1168–1187. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van der Loos H, Woolsey TA. Somatosensory cortex: Structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- 96.Tolner EA, et al. Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. J. Neurosci. 2012;32:692–702. doi: 10.1523/JNEUROSCI.1538-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seelke AMH, et al. The emergence of somatotopic maps of the body in S1 in rats: The correspondence between functional and anatomical organization. PLOS ONE. 2012;7:e32322. doi: 10.1371/journal.pone.0032322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blumberg MS, et al. Twitching in sensorimotor development from sleeping rats to robots. Curr. Biol. 2013;23:R532–R537. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khazipov R, et al. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 100.Kaas JH, Rothemund Y. Reorganization of somatosensory and motor cortex following peripheral nerve or spinal cord injury in primates. In: Lomber SG, Eggermont JJ, editors. Reprogramming the cerebral cortex: Plasticity following central and peripheral lesions. Oxford University Press; 2006. pp. 285–296. [Google Scholar]
- 101.Wall JT, et al. Functional reorganization in somatosensory cortical areas 3b and 1 of adult monkeys after median nerve repair: Possible relationships to sensory recovery in humans. J. Neurosci. 1986;6:218–233. doi: 10.1523/JNEUROSCI.06-01-00218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Florence SL, et al. Central reorganization of sensory pathways following peripheral nerve regeneration in fetal monkeys. Nature. 1996;381:69–71. doi: 10.1038/381069a0. [DOI] [PubMed] [Google Scholar]
- 103.Zembrzycki A, et al. Sensory cortex limits cortical maps and drives top-down plasticity in thalamocortical circuits. Nat. Neurosci. 2013;16:1060–1067. doi: 10.1038/nn.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]