Table 3.
CuBr2/1ox-catalyzed aerobic oxidative cross-coupling of benzylamine 2 a or aminomethylcyclopropane 2 n with o-aminoanilines 3[a]
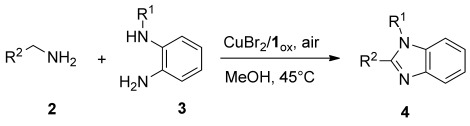 | ||||
|---|---|---|---|---|
| Entry | R2 | R1 | Benzimidazole Product4 | Yield [%][c] |
| 1 | phenyl | methyl | 4 t | 81 |
| 2 | phenyl | p-chlorophenyl | 4 u | 84[d] |
| 3[b] | cyclopropyl | methyl | 4 v | 73[e] |
| 4[b] | cyclopropyl | p-chlorophenyl | 4 w | 72[d,e] |
[a] The reactions were carried out using equimolar amounts of primary amine 2 a (or 2 n) and o-aminoaniline 3 on a 1.25 mmol scale, in the presence of 4 mol % of 1red and 0.4 mol % of CuBr2, in 25 mL of MeOH, under ambient air for 24 h. After 6 h, an additional aliquot of 1red (2 mol %) was added; [b] T=60 °C; [c] yield of isolated product; [d] yield after 48 h; [e] as volatile alkylamine 2 n was lost at 60 °C, an additional 0.5 equivalent of alkylamine was added after 6 h (entries 3 and 4) and after 24 h (entry 4).
