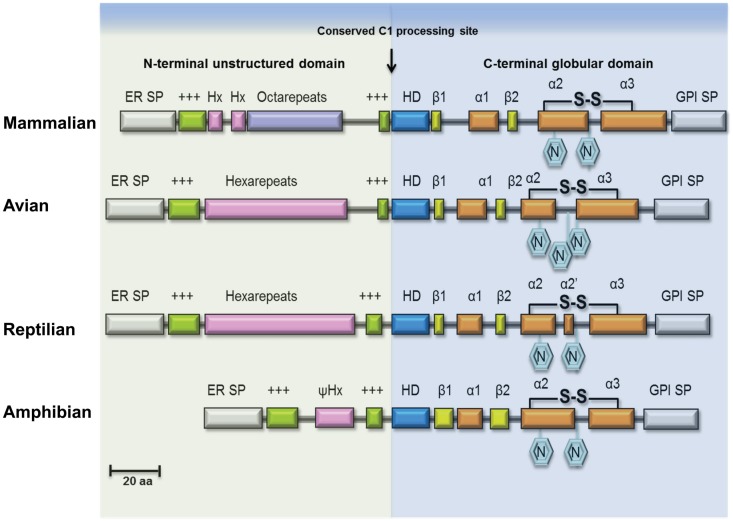
Figure 1.
Structural features of PrPC in vertebrate lineages. Schematic illustration of major structural features of PrPC in terrestrial vertebrates. The ER transfer signal peptide (ER SP) and the glycophosphatidylinositol-anchor attachment signal peptide (GPI SP) are removed during maturation of the primary translation product, followed by N-glycosylation and cysteine bridge formation in the C-terminus. The N-terminal domain is an intrinsically disordered region (IDR) of variable length containing repeated sequence elements (pink/purple) flanked by two positively charged amino-acids motifs (green). In mammalian PrPC, this region contains a number of glycine-rich octapeptides or nonapeptides, preceded by two hexapeptides, while in avian and reptilian PrPC it is comprised entirely of hexapeptides, and in amphibian PrPC only a very short pseudo-hexapeptide (ψHx) sequence is present. A highly conserved hydrophobic, alanine-rich motif (HD) at the center is characteristic for PrPC. Proteolytic cleavage of PrPC, known as α-cleavage (arrow) occurs N-terminal to the HD-motif at the boundary between the IDR domain and the globular C-terminal domain thus generating the fragments PrP-N1 and PrP-C1 of PrPC (not shown). The globular domain consists in most PrPs of three helices (α1, α2, α3) and a β-sheet formed by two short β-strands (β1, β2).