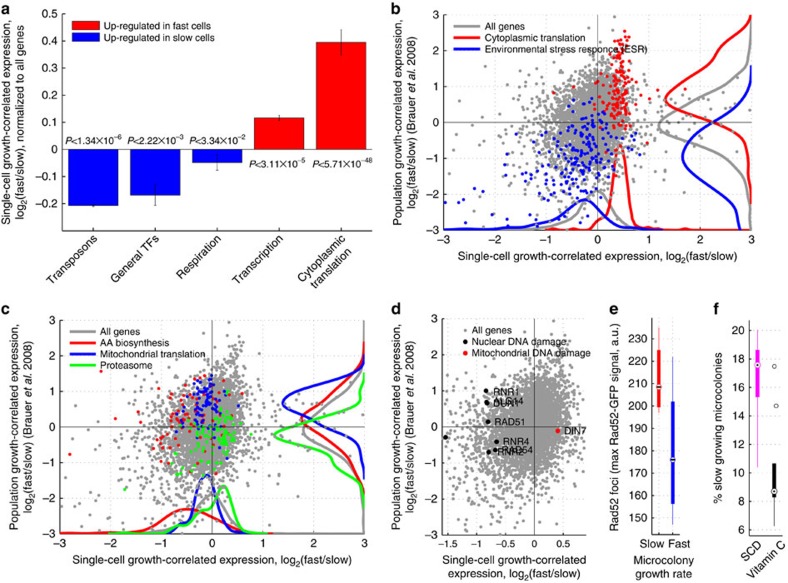
Figure 3. Transcriptional profiles of mean and subpopulation growth.
(a) Bar-plot showing mean and standard expression of all genes in each functional group of genes upregulated in the slow- (blue) or fast (blue)-growing subpopulations. (b–d) Growth-correlated expression from slow- and fast-growing subpopulations (FitFlow, x axis) are compared with expression differences from growth rate varied in nutrient limited chemostats (y axis). (b) Scatter-plot the correlation of gene expression between subpopulation growth and mean population growth. Ribosomal genes (red) and stress genes (blue) are, respectively, up- and downregulated both in subpopulation (x axis, paired ks-tests Pred=3.36 × 10−67; Pblue=4.02 × 10−21) and mean population (y axis, paired ks-tests Pred=2.45 × 10−36; Pblue=8.26 × 10−50) fast growth. (c) Scatter-plot highlighting genes for which subpopulation growth is anti-correlated with mean population growth. Amino-acid biosynthesis (red) and mitochondrial translation (blue) are downregulated in the fast subpopulation (paired ks-tests Pred=1.03 × 10−12; Pblue=3.31 × 10−04) but upregulated in mean population fast growth (paired ks-tests Pred=3.30 × 10−04; Pblue=2.64 × 10−16), while the proteasome (green) is upregulated in the fast subpopulation (paired ks-test P=2.25 × 10−06) but downregulated in mean population fast growth (paired ks-test P=4.69 × 10−07). (d) DNA damage genes (black points) are upregulated in the slow subpopulation (x axis, paired ks-test P=4.73 × 10−07), but are not correlated with average population growth rate differences (y axis, paired ks-test P=0.83). DIN7 (red point) is involved in mitochondrial DNA damage repair, and is the only DNA damage related gene that is not upregulated in the slow subpopulation. (e) Time-lapse microscopy shows that cells from the slow-growing subpopulation have Rad52–GFP foci. Foci were measured as the maximum Rad52–GFP signal in the nucleus (y axis) and growth (x axis) as the microcolony growth rate where slow and fast cells represent the slowest 25% and fastest 75%, respectively (t-test, P=0.02). (f) Addition of the antioxidant vitamin C reduced the fraction of slow-growing microcolonies (t-test, P=0.005).