Figure 2. Regulation of PEDF expression, function and degradation.
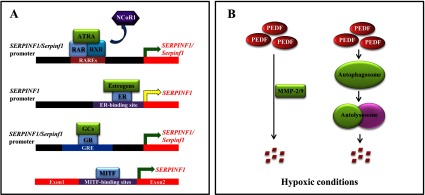
(A) Transcriptional regulation of human SERPINF1/rodent Serpinf1. Six RAREs are located at −1000 to −1 bp of the SERPINF1/Serpinf1 promoter region. In the presence of ATRA, RAR/RXR heterodimers bind to RAREs, dissociate from NCoR1, and recruit co-activators to activate SERPINF1/Serpinf1 transcription. In the absence of agonists, RAR/RXR heterodimers associate with NCoR1, which suppresses SERPINF1/Serpinf1 transcription. At least one ER-binding site is located at −864/+63 bp of the SERPINF1 promoter. Oestrogens induce ERs to form either heterodimers or homodimers to bind to the ER-binding site, triggering/suppressing SERPINF1 transcription depending on cell types and tissues. In addition, at least one GRE is found at −1721/+38 bp of the SERPINF1/Serpinf1 promoter. GCs bind to GRs and promote GR nuclear translocation, which then initiates SERPINF1/Serpinf1 transcription via the promoter region GRE. Three micropthalmia-associated transcription factor (MITF)-binding regions are identified within the first intron of the SERPINF1 gene. MITF binds to the MITF-binding sites and up-regulates the transcription of SERPINF1. (B) Down-regulation of PEDF by hypoxia. Under hypoxic conditions, expression and activities of MMP-2/9 are increased, which promote the degradation of PEDF protein. In addition, a HIF-1-independent pathway to degrade PEDF was also reported. Hypoxia stimulates the autophagosome to down-regulate PEDF levels. Continuous green arrows represent gene transcription activation, whereas broken yellow arrows illustrate either activation or suppression of gene transcription depending on context. GCs, glucocorticoid/glucocorticoid analogues; GR, glucocorticoid receptor; GRE, glucocorticoid response element; NCoR1, nuclear receptor co-repressor 1.
