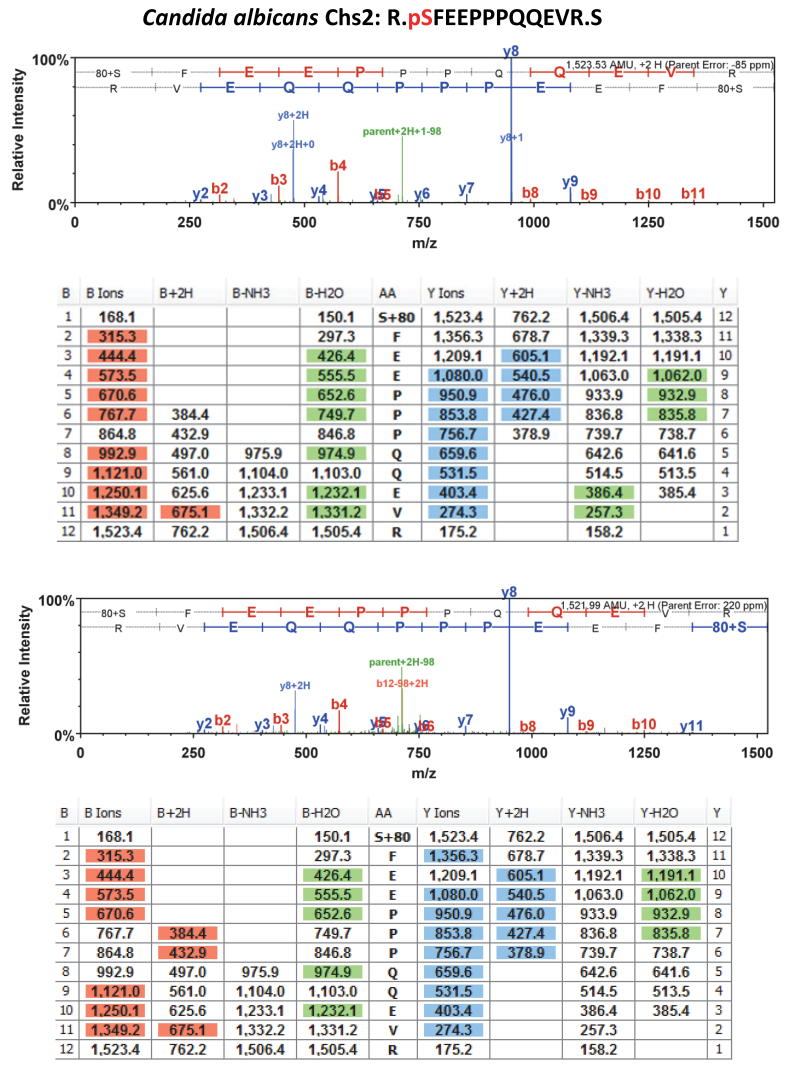
Supplementary Fig. S1.
Identification of a phosphorylation site on Chs2. Sequencing of a phosphopeptide from Chs2 by nano-electrospray ionisation tandem mass spectrometry (nano-ESI-MS/MS) showed that the serine residue at the N-terminal end of the peptide is phosphorylated. Phosphopeptide preparation and mass spectrometry were performed as described previously (Lenardon et al., 2010a). Data were exported as SRF files into Scaffold (www.proteomesoftware.com) to produce the annotated spectra. Two independent spectra obtained for the same phosphopeptide are shown. In each example, the parent ion after a neutral loss of phosphoric acid is labelled parent+2H-98 (green). The individual y – (blue) and b – (red) ions are labelled on the spectra.